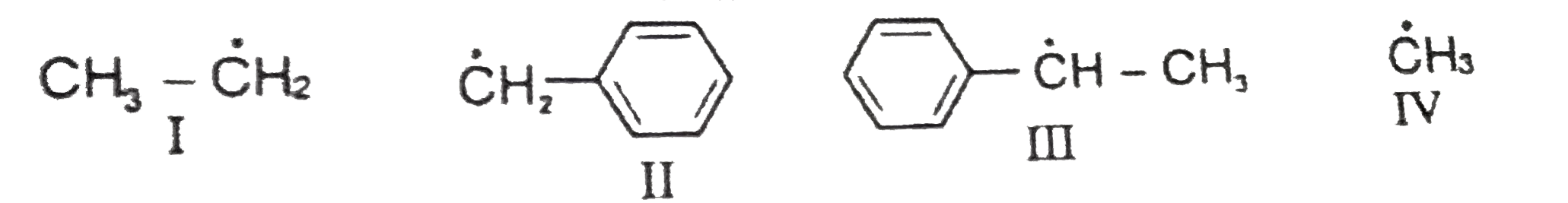
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY II
RESONANCE|Exercise Part-II Only One Option Correct Type|44 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE|Exercise Part-III: Match the Column|2 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE|Exercise Part-III: Section-5: Matching List Type|1 VideosGASEOUS STATE
RESONANCE|Exercise PHYSICAL CHMISTRY (Gaseous State)|47 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE|Exercise PART-III : PRACTICE TEST-19|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-GENERAL ORGANIC CHEMISTRY II-Exercise-1
- Arrange the following in decreasing order of stability
Text Solution
|
- Arrange the following in decreasing order of stability
Text Solution
|
- Range the following free radicals in increasing order of their stabili...
Text Solution
|
- Arrange the following free radicals in decreasing order of stability:
Text Solution
|
- Arrange the following carbocations in decreasing order of their stabil...
Text Solution
|
- Which of the following carbocation is more stable and why?
Text Solution
|
- Draw the structure of P and Q.
Text Solution
|
- Compare the basic strength of the following compounds: underset((I))...
Text Solution
|
- Compare the basic strength of the following compound:
Text Solution
|
- Which of the following group is most basic in the given compounds"
Text Solution
|
- Which of the following is a stronger base? Give reason to justify your...
Text Solution
|
- Which 'H' atom is most acidic in the following compounds.
Text Solution
|
- Arrange the following in decreasing order of acidity
Text Solution
|
- The given compound X= is a strong acid. Justify this statement.
Text Solution
|
- Which of the following reactions is/are feasible? (a) CH(3)COOH+HC"O...
Text Solution
|
- Which of the following reaction is feasible?
Text Solution
|
- Which of the following acids (given below) react with NaHCO(3) and lib...
Text Solution
|
- Which of the following compounds are exhibit tautomerism?
Text Solution
|
- Write the tautomers of the following compounds.
Text Solution
|
- Monocarbonyl compounds have very small percentage enol form at equilib...
Text Solution
|