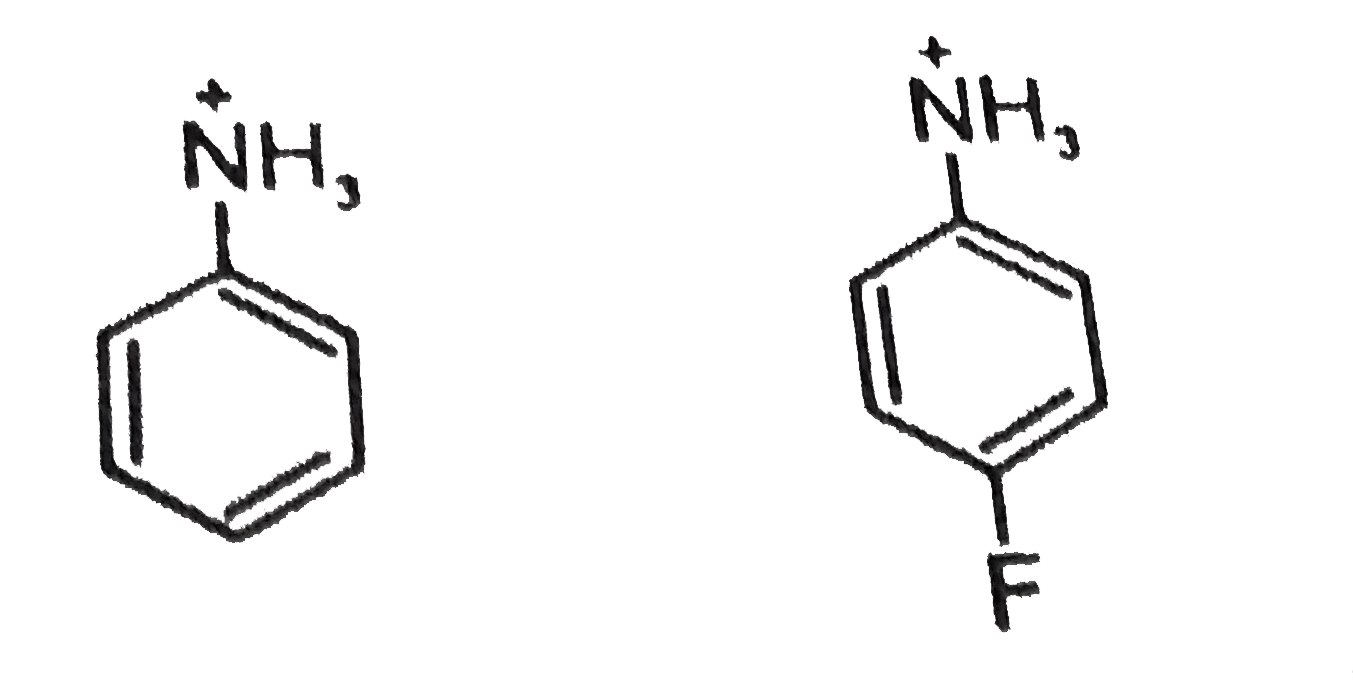
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY II
RESONANCE|Exercise Exercise-3 Part:II JEE(MAIN)/AIEEE PROBLEMS (PREVIOUS YEARS)|18 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE|Exercise Exercise-3 Part:II JEE(MAIN) ONLINE PROBLEMS|16 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE|Exercise Part-IV : Comprehension|14 VideosGASEOUS STATE
RESONANCE|Exercise PHYSICAL CHMISTRY (Gaseous State)|47 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE|Exercise PART-III : PRACTICE TEST-19|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-GENERAL ORGANIC CHEMISTRY II-Exercise-3 Part:I JEE(Advanced)/IIT-JEE Problems (Previous Years)
- Which of the following acid has the lowest value of acid dissociation ...
Text Solution
|
- Match the K(a) values:
Text Solution
|
- A compound A of molecular formula C(9)H(7)O(2)Cl exists in keto form a...
Text Solution
|
- . The product A will be-
Text Solution
|
- What is the acidity order of x,y & z ?
Text Solution
|
- Which one of the following two compounds is the stronger acid ? Explai...
Text Solution
|
- The products will be :
Text Solution
|
- Benzenesulphonic acid and para nitrophenol react with NaHCO(3) separat...
Text Solution
|
- The correct stability order for the following species is :
Text Solution
|
- The correct acidity order of the following is :
Text Solution
|
- In the following carbocation, H//CH(3) that is most likely to migrate ...
Text Solution
|
- The total number of basic groups in the following form of lysine is :
Text Solution
|
- Among the following compounds, the most acidic is :
Text Solution
|
- The carboxyl functional group (-COOH) is present in
Text Solution
|
- The compound that does NOT liberate CO(2) , on treatment with aqueous ...
Text Solution
|
- The correct order of acidity for the following compounds is
Text Solution
|
- The order of basicity among the following compounds is
Text Solution
|