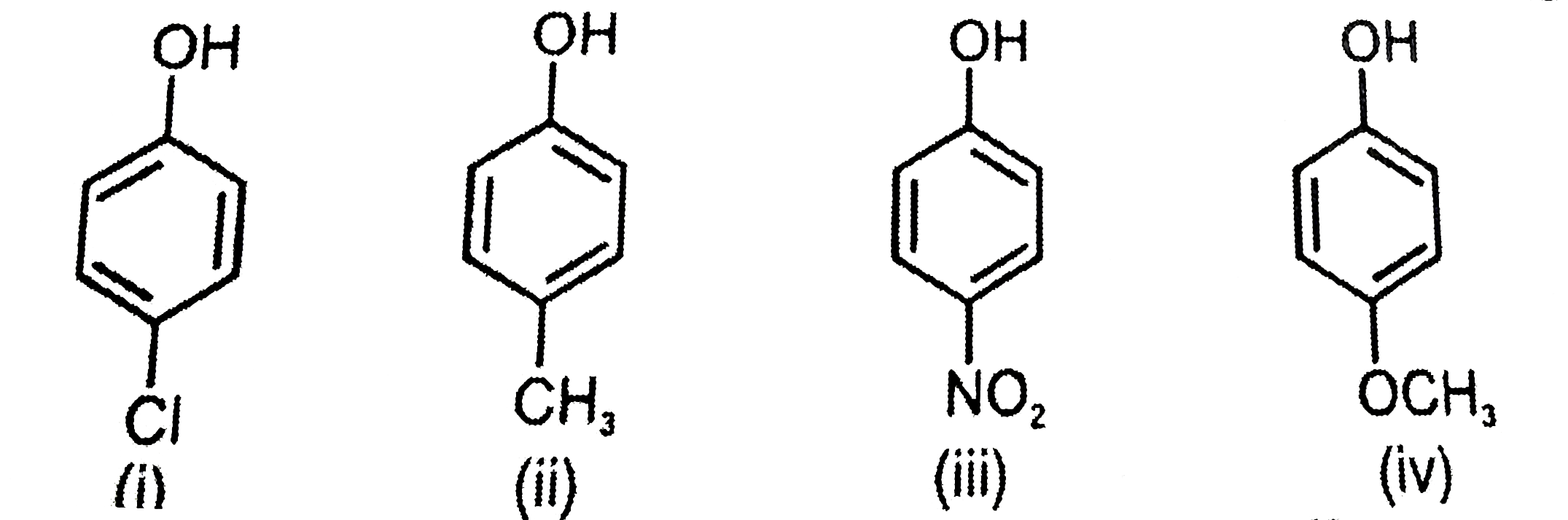
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY II
RESONANCE|Exercise Part-II: NSEC (Stage-I)|46 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE|Exercise Part-III: Practice Test-2 (IIT-JEE (Advanced Pattern))|16 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE|Exercise Exercise-3 Part:II JEE(MAIN) ONLINE PROBLEMS|16 VideosGASEOUS STATE
RESONANCE|Exercise PHYSICAL CHMISTRY (Gaseous State)|47 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE|Exercise PART-III : PRACTICE TEST-19|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-GENERAL ORGANIC CHEMISTRY II-(APSP) Part-I:Practice Test-1
- Which of the following would produce effervesence with sodium bicarbon...
Text Solution
|
- Select correct statement from the following:
Text Solution
|
- Arrange the following compounds in order of decreasing acidity :
Text Solution
|
- The order of decreasing basicity in the four halide ions is :
Text Solution
|
- Correct order of acidic strength :
Text Solution
|
- Which of the following is incorrect about the given molecules
Text Solution
|
- Which of the following is the most stabilized carbocation ?
Text Solution
|
- Which one among the following is the least basic:
Text Solution
|
- Which is most basic in aqueous solution ?
Text Solution
|
- Stability order of given anions is :
Text Solution
|
- Which is less basic than benzyl carbanion ?
Text Solution
|
- The correct order of decreasing acid strength of trichloroacetic acid ...
Text Solution
|
- Base strength is in the order of (i) CH(3)barCH(2)" "(ii) H(2)C=...
Text Solution
|
- The order of stability of following carbocation :
Text Solution
|
- The most stable carbocation is:
Text Solution
|
- Pyridine is less basic than triethylamine because :
Text Solution
|
- Which is the following phenol has lowest pK(a) ?
Text Solution
|
- Which is most basic among the followings ?
Text Solution
|
- Assertion: The pK(a) of acetic acid is lower than that of phenol Rea...
Text Solution
|
- The order of stability of the following tautomeric compounds is : CH...
Text Solution
|