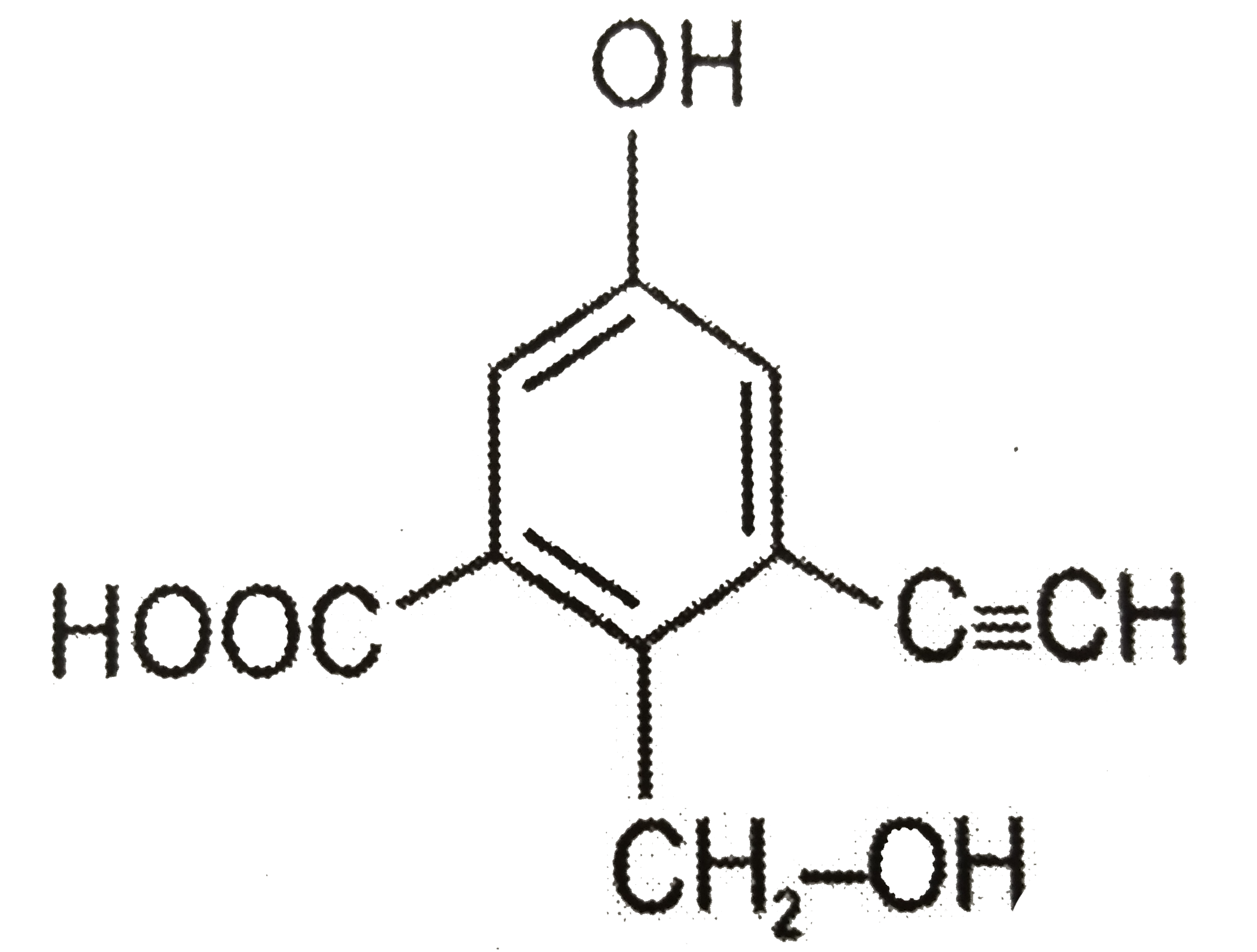
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRY
RESONANCE|Exercise Section(D)|9 VideosSTRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRY
RESONANCE|Exercise Part-II Only one correct option type|1 VideosSTRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRY
RESONANCE|Exercise Section-B|5 VideosSOLID STATE
RESONANCE|Exercise Part- IV|22 VideosTHERMODYNAMIC & THERMOCHEMISTRY
RESONANCE|Exercise PHYSICAL CHEMITRY (THERMODYNAMIC & THERMOCHEMISTRY)|62 Videos
Similar Questions
Explore conceptually related problems