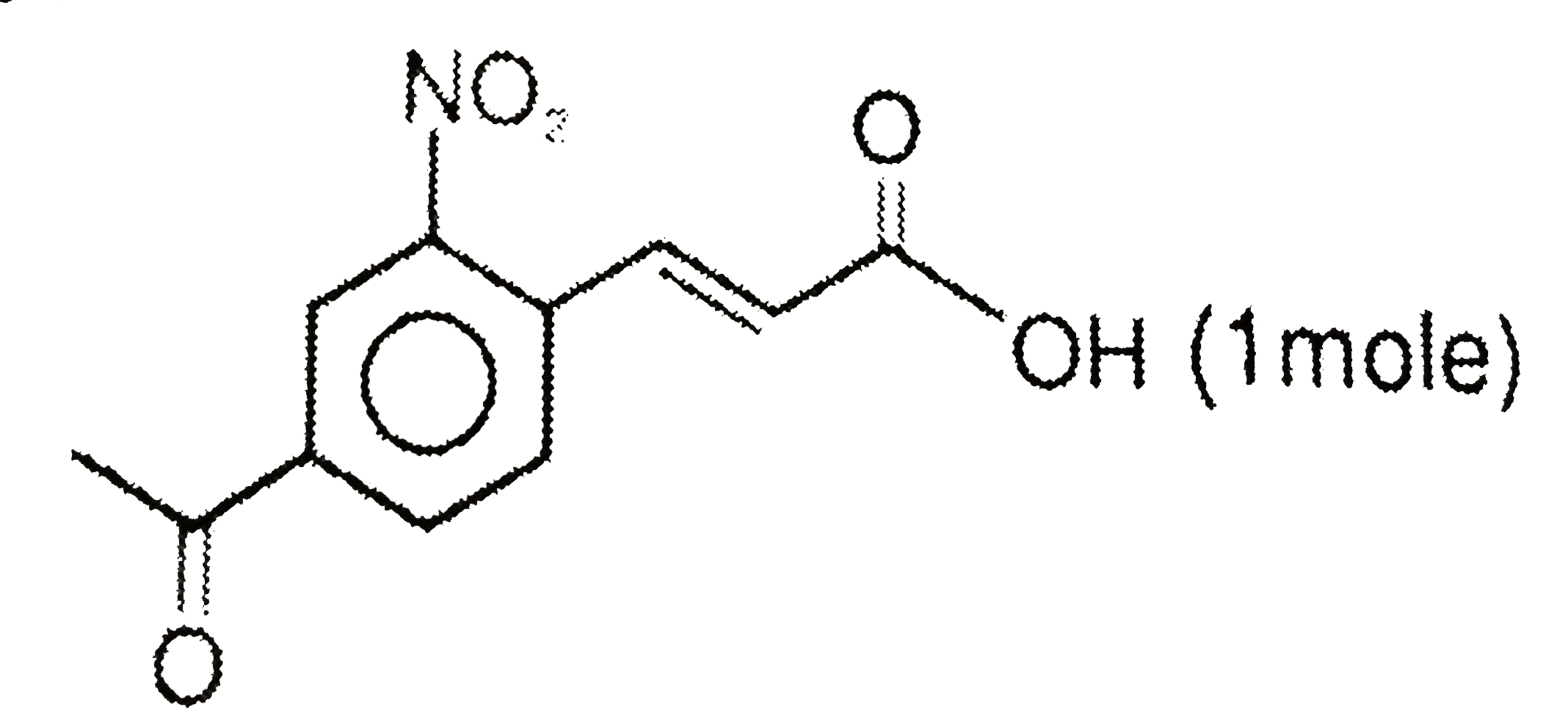
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRY
RESONANCE|Exercise Section-D|13 VideosSTRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRY
RESONANCE|Exercise Section-E|8 VideosSTRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRY
RESONANCE|Exercise SectionB|7 VideosSOLID STATE
RESONANCE|Exercise Part- IV|22 VideosTHERMODYNAMIC & THERMOCHEMISTRY
RESONANCE|Exercise PHYSICAL CHEMITRY (THERMODYNAMIC & THERMOCHEMISTRY)|62 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRY-Section-C
- When one mole of the given compound reats with sodium metal then how m...
Text Solution
|
- Compound X is
Text Solution
|
- Identify X
Text Solution
|
- Ammonical AgNO(3) gives white ppt after reaction with any compound the...
Text Solution
|
- Which of the following compound gives red ppt with CuCl(2)//NH(4)OH ?
Text Solution
|
- Identify the hydrocarbon having molecular formula C(5)H6 which gives w...
Text Solution
|