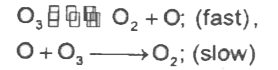
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The decomposition of ozone is believed to occur by the mechanism : ...
Text Solution
|
- For the reaction 2N(2)O(5)rarr 4NO(2)+O(2) , if rate formation of O(2)...
Text Solution
|
- Assertion: Equivalent mass of ozone in the change O(3) rarr O(2) is 8....
Text Solution
|
- Determining the rate law from a mechanism with an initial slow step: O...
Text Solution
|
- Rate law for ozone layer depletion is, (d[O(3)])/(dt)=(K[O(3)]^(2))/...
Text Solution
|
- The chemical reaction O(3) to 3O(2) proceeds as O(3) hArr O(2)+O("fast...
Text Solution
|
- In the decomposition of ozone, 2 O(3)to3O(2) , order with respect to O...
Text Solution
|
- A possible mechanism for the conversion for ozone to oxygen in the upp...
Text Solution
|
- Consider the proposed mechanism for the destruction of ozone in the st...
Text Solution
|