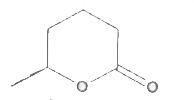
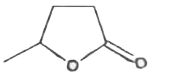
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise Section (II) [Jee mains]: With Single Correct Choice|78 VideosGENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise MORE THAN ONE CORRECT CHOICES|24 VideosGENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|14 VideosGASEOUS STATE
FIITJEE|Exercise MATCHING LIST TYPE QUESTION|1 VideosHYDROCARBONS
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTION|9 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-GENERAL ORGANIC CHEMISTRY-Section (I) [Jee mains]: With Single Correct Choice
- alpha-amino acid may be prepared by
Text Solution
|
- Propanoic acid on warming with Br(2) in presence of red P gives
Text Solution
|
- MeCH(OH)CH(2)CH(2)COOH when heated produces
Text Solution
|
- Between acids K(a) is more for
Text Solution
|
- Among the given compounds, the most susceptible to nucleophilic attack...
Text Solution
|
- Reagent A used in this change is
Text Solution
|
- Which among the following is most acidic?
Text Solution
|
- Identify the correct statement
Text Solution
|
- The product P may be
Text Solution
|
- Which will most readily precipitate with AgNO(3) ?
Text Solution
|
- PbCl(2) is ionic whereas PbCl(4) is covalent. This is due to
Text Solution
|
- X and Y are.
Text Solution
|
- In the following compounds the electrophilic substitution in the ...
Text Solution
|
- End product of the following sequence of reactions is
Text Solution
|
- Which of the following compound is not aromatic?
Text Solution
|
- can exhibit
Text Solution
|
- Which of the following compounds have more than one type of hybridisat...
Text Solution
|
- Between maleic acid fumaric acids, K(a(1)) is more for
Text Solution
|
- which H is abstracted easily?
Text Solution
|
- The following compound can exhibit
Text Solution
|