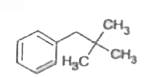
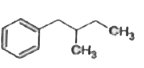
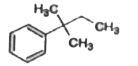
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise MORE THAN ONE CORRECT CHOICES|24 VideosGENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise COMPREHENSION TYPE|18 VideosGENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise Section (I) [Jee mains]: With Single Correct Choice|100 VideosGASEOUS STATE
FIITJEE|Exercise MATCHING LIST TYPE QUESTION|1 VideosHYDROCARBONS
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTION|9 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-GENERAL ORGANIC CHEMISTRY-Section (II) [Jee mains]: With Single Correct Choice
- Arrange the following in the decreasing order of reactivity towards el...
Text Solution
|
- Predict the product
Text Solution
|
- When benzene reacts with neopentyl bromide CH(3)-underset(CH(3))unders...
Text Solution
|
- For the reaction What is the product P?
Text Solution
|
- The above conversion can be done by
Text Solution
|
- When phenol is treated with CHCl(3) and NaOH, followed by acidificatio...
Text Solution
|
- Identify the final pr(Z) in the following sequence of reactions.
Text Solution
|
- An alcohol, on dehydration produces an alkene which on ozonolysis yiel...
Text Solution
|
- Eacof nucleophilic substitution among these compounds will be in the o...
Text Solution
|
- The cannizzaro reaction of PhCOCHO forms the product(s)
Text Solution
|
- Complete the following reaction
Text Solution
|
- CH(3)-CH=CH-CH=CH-overset(O)overset(||)(C)-H overset("dil. "OH^(-))to ...
Text Solution
|
- The carboxylic acid undergoing decarboxylation most readily is/are
Text Solution
|
- Identify the final product PhCH(3) underset(H(2)SO(4))overset(HNO(3)...
Text Solution
|
- The transformation Can be brought about by reducing the reactant ...
Text Solution
|
- What will be the major product?
Text Solution
|
- (I) Cl-underset(Cl)underset(|)overset(Cl)overset(|)(C^(3))-overset(2)(...
Text Solution
|
- H(3)C-CH(2)-underset(Br)underset(|)(C)H-CH(3) overset(NaNH(2))to Whi...
Text Solution
|
- H(3)C-underset(CH(3))underset(|)overset(CH(3))overset(|)(C)-underset(O...
Text Solution
|
- A will be
Text Solution
|