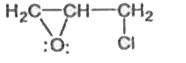
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise MORE THAN ONE CORRECT CHOICES|24 VideosGENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise COMPREHENSION TYPE|18 VideosGENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise Section (I) [Jee mains]: With Single Correct Choice|100 VideosGASEOUS STATE
FIITJEE|Exercise MATCHING LIST TYPE QUESTION|1 VideosHYDROCARBONS
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTION|9 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-GENERAL ORGANIC CHEMISTRY-Section (II) [Jee mains]: With Single Correct Choice
- H(3)C-underset(CH(3))underset(|)overset(CH(3))overset(|)(C)-underset(O...
Text Solution
|
- A will be
Text Solution
|
- CH(2)=CH-CH(2)-Cl overset(HOC l)to (X) is
Text Solution
|
- H(3)C-Mg-Br+H(2)C=CH-overset(O)overset(||)(C)-H overset(H(3)O^(+))to ...
Text Solution
|
- H(3)C-CH(2)-underset(O)underset(||)(C)-CH(3)underset(D(2)O)overset(NaB...
Text Solution
|
Text Solution
|
- Greatest amount of hydration is in
Text Solution
|
- ?
Text Solution
|
- m-nitro benzoic acid can be obtained by
Text Solution
|
Text Solution
|
- , X is
Text Solution
|
- Product will be
Text Solution
|
- Compound 'X' has molecular formula C(4)H(8)O(3). It evolves CO(2) with...
Text Solution
|
Text Solution
|
- The product is
Text Solution
|
- Which of the following will most readily dehydrated in acidic conditio...
Text Solution
|
- Oxidation product of 1,2-cyclopentane-diol with HlO(4) is
Text Solution
|
- X will be
Text Solution
|
- . Product X is
Text Solution
|
- Which among the following compounds are oxidised by NalO(4) Selec...
Text Solution
|