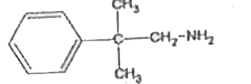
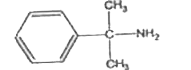
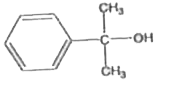
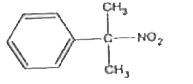
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise MORE THAN ONE CORRECT CHOICES|24 VideosGENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise COMPREHENSION TYPE|18 VideosGENERAL ORGANIC CHEMISTRY
FIITJEE|Exercise Section (I) [Jee mains]: With Single Correct Choice|100 VideosGASEOUS STATE
FIITJEE|Exercise MATCHING LIST TYPE QUESTION|1 VideosHYDROCARBONS
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTION|9 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-GENERAL ORGANIC CHEMISTRY-Section (II) [Jee mains]: With Single Correct Choice
- Major product is
Text Solution
|
- What is the major product in the reaction?
Text Solution
|
- Compound (A) C(5)H(10)O forms a phenyl hydrogen and gives negative Tol...
Text Solution
|
- 2-methyl-2-phenyl propanamide when reacted with bromine and NaOH forms...
Text Solution
|
- Potassium pthalimide on reaction wiith (A) followed by hydrolysis form...
Text Solution
|
- Which of the following will give more enol contents ?
Text Solution
|
- The product is
Text Solution
|
- In the reaction of p-chlorotoluene with KNH(2) in liq. NH(3), the majo...
Text Solution
|
- Certain chemical tests were perfoemd on the pain-relief drug ibuprofen...
Text Solution
|
- A and B are
Text Solution
|
- Number of C-C bonds present in anthracene are
Text Solution
|
- The product(s) of the Hofmann exhaustive methylation of the following ...
Text Solution
|
- In the following groups (1) -OA c (2) -OM e (3) -OSO(2)Me (4) ...
Text Solution
|
- Which allylic carbocation is the most stable carbocation ?
Text Solution
|
- What is Z in the following sequence of reactions? Phenolunderset("du...
Text Solution
|
- Under Wolff Kischner reaction conditions, the coversation which may be...
Text Solution
|
- Which of the following is the strongest acid?
Text Solution
|
- RNA is different from DNA< because RNA contains:
Text Solution
|
- Insulin contains:
Text Solution
|
- A decapiptide (Mol. Wt. 796) on complete hydrolysis gives glycine (Mol...
Text Solution
|