Text Solution
Verified by Experts
Topper's Solved these Questions
PREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTS
FIITJEE|Exercise SOLVED PROBLEMS (SUBJECTIVE)Level-I|29 VideosPREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTS
FIITJEE|Exercise SOLVED PROBLEMS (OBJECTIVE)|27 VideosPERIODIC PROTERTIES OF ELEMENTS
FIITJEE|Exercise COMPREHENSION|13 VideosQUALITATIVE ANALYSIS
FIITJEE|Exercise Single interger answer type|3 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-PREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTS- SINGLE INTEGER ANSWER TYPE QUESTIONS
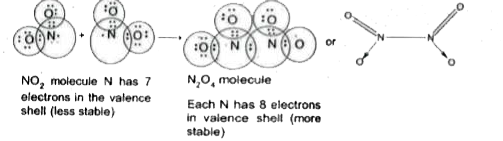
- why does NO(2) dimerise?
Text Solution
|
- A 1.10 g sample of copper ore is dissolved and the Cu^(2+) of is treat...
Text Solution
|
- 10 ml of H(2)O(2) solution on treatment with Kl and titration of liber...
Text Solution
|
- What is the oxidation number of Cr in Cro 5. The silicate anion in the...
Text Solution
|
- Both Cr(2)O(7)^(2-) and MnO(4)^(-) solutions can be used to titrate Fe...
Text Solution
|
- The difference in the oxidation numbers of the two types of sulphur at...
Text Solution
|
- Reaction of Br(2) with Na(2)CO(3) in aqueous solution gives sodium bro...
Text Solution
|
- Among the following, the number of compounds that can react with PCl(5...
Text Solution
|
- Find the no. of gaseous species (including water vapours) obtained on ...
Text Solution
|