Text Solution
Verified by Experts
Topper's Solved these Questions
PREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTS
FIITJEE|Exercise ASSIGNMENT PROBLEMS (SUBJECTIVE) LEVEL-I(short-Answer tpye question)|5 VideosPREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTS
FIITJEE|Exercise ASSIGNMENT PROBLEMS (SUBJECTIVE) LEVEL-I(Give reasons: )|5 VideosPREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTS
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|2 VideosPERIODIC PROTERTIES OF ELEMENTS
FIITJEE|Exercise COMPREHENSION|13 VideosQUALITATIVE ANALYSIS
FIITJEE|Exercise Single interger answer type|3 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-PREPARATION AND PROPERTIES OF COMPOUNDS AND GENERAL DISCUSSION OF S-AND P-BLOCK ELEMENTS-Exercise
- Which oxide of N is formed when NH(4)NO(3), is heated?
Text Solution
|
- What type of pi-bonding is present in the compound of type POX(3) ?
Text Solution
|
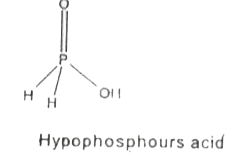
- How do you account for the reducing behaviour of H(3)PO(2)? Why are pe...
Text Solution
|
- What is the geometry of [Cu(H(2)O)(4)]^(2+) ?
Text Solution
|
- (i)Magnetic moment of [Ag(CN)(2)] is zero .How many unpaired electrons...
Text Solution
|
- What would be electronic configuration of the following in the absence...
Text Solution
|
- Arrango He, Li, Be, C. B ,N,OF ,Ne Non increasing second ionization en...
Text Solution
|
- Identity (A), (B),(C), (D) and (E) in the following: Alkali.metal (A)...
Text Solution
|
- Match the following column with column
Text Solution
|
- What are (A), (B), (C) and (D)
Text Solution
|
- Sodium forms two oxides (A) and (B) having 25.81 % and 41.03% oxygen r...
Text Solution
|
- 3 mof of mixture of NaHCO(3) and Na(2)CO(3) is strongly heated at 200°...
Text Solution
|
- 3 mof of mixture of NaHCO(3) and Na(2)CO(3) is strongly heated at 200°...
Text Solution
|
- Predict whether Tit will disproportionate in aqueous solution, given t...
Text Solution
|
- Why the first ionisation energy of carbon atom is greater than that of...
Text Solution
|
- PbCI(2) exists white PbBr and PbI do not exist.
Text Solution
|
- Which among the following oxides of lead is strong oxidizing agent? Pb...
Text Solution
|
- Account for the following : (i)The experimentally determined N-F bon...
Text Solution
|
- PF(5) is known but NF(5) is not. Explain.
Text Solution
|
- The electronegativity of both nitrogen and chlorine is the same. But n...
Text Solution
|