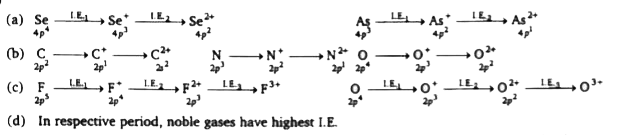
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC PROPERTIES
VK JAISWAL|Exercise MATCH THE COLUMN|13 VideosPERIODIC PROPERTIES
VK JAISWAL|Exercise ASSERTION-REASON TYPE QUESTIONS|35 VideosPERIODIC PROPERTIES
VK JAISWAL|Exercise Level 1|198 Videosp-BLOCK ELEMENTS
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|36 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|5 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-PERIODIC PROPERTIES-Level 2
- The third ionization enthalpy is maximum for
Text Solution
|
- Consider the following ionisation reactions: If monovalent positi...
Text Solution
|
- The incorrect statement is:
Text Solution
|
- First three ionisation energies (in kJ/mol) of three representative el...
Text Solution
|
- Which of the following statement is correct regarding following specie...
Text Solution
|
- The correct order of increasing electron affinity of the following ele...
Text Solution
|
- The second electron gain enthalpies (in kJ mol^(-1)) of oxygen and sul...
Text Solution
|
- Which of the following statement is correct?
Text Solution
|
- Which of the following statements is incorrect?
Text Solution
|
- The formation of the oxide ion O(g)^(2-) requires first an exothermic ...
Text Solution
|
- In which of the following energy is abosrbed?
Text Solution
|
- The electron affinity of the following elements can be arranged:
Text Solution
|
- In which of the following arrangements, the order is not correct accor...
Text Solution
|
- Which of the following statements is/are wrong?
Text Solution
|
- Consider the following conversions: (i) O((g))+e^(-) to O((g))^(-),D...
Text Solution
|
- {:("Element","Electronegative value"),(W,2.7),(X,2.1),(Y,0.8),(Z,3.4):...
Text Solution
|
- In the compound M-O-H, the M-O bond will be broken if:
Text Solution
|
- Aqueous solutions of two compounds M(1)-O-H and M(2)-O-H are prepared ...
Text Solution
|
- If the ionisation enthalpy and electron gain enthalpy of an element ar...
Text Solution
|
- Consider the following statement: (I) The radius of an anion is larg...
Text Solution
|