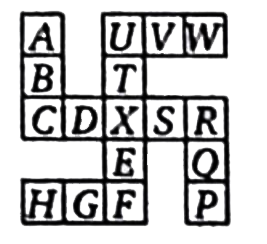A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL-PERIODIC PROPERTIES-ASSERTION-REASON TYPE QUESTIONS
- Consider the following representation based on long form of periodic t...
Text Solution
|
- Assertion: In CsF, salt, size of Cs^(+) is slightly higher than size o...
Text Solution
|
- Assertion :- First electrons affinity fo all element is positive. Re...
Text Solution
|
- Assertion: Helium has the highest value of ionisation energy among all...
Text Solution
|
- Assertion: F^(-) ion has highest hydrated radius among the other halid...
Text Solution
|
- Assertion: magnitude of electron gain enthalpy of oxygen is less than ...
Text Solution
|
- Assertion: Formation of Cl^(-) ion is exothermic wheres O^(2-) ion for...
Text Solution
|
- Assertion: The electron gain enthalpy of N is +ve while that of P is -...
Text Solution
|
- Assertion: The formation of F((g))^(-) from F((g)) is exothermic, wher...
Text Solution
|
- Statement-1: Na^(+) and AI^(3+) are isoelectronic but the magnitude of...
Text Solution
|
- Assertion: The third period contains only 8 electrons and not 18 like ...
Text Solution
|
- Statement-I : Cs and F combines violently to form CsF. Statement-II :...
Text Solution
|
- Statement-1: Second electron gain enthalpy of halogens is always posit...
Text Solution
|
- Assertion: F atom has less electron afffinity than Cl atom. Reason: ...
Text Solution
|
- Assertion: Among the halogens bond energy of F(2) is minimum. Reason...
Text Solution
|
- Assertion: The first ionization energy of Be is greater than that of B...
Text Solution
|
- Assertion : Noble gases have highest ionisation enthalpies in their re...
Text Solution
|
- Assertion: Helium and beryllium have similar outer electronic configur...
Text Solution
|
- Assertion: The first ionisation enthalpy of aluminium is lower than th...
Text Solution
|
- Assertion: In CsF, salt, size of Cs^(+) is slight higher than size of ...
Text Solution
|
- Assertion: First electron affinity of all elements is positive. Reas...
Text Solution
|