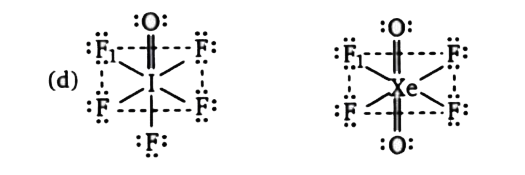
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise ONE OR MORE ANSWER IS/ARE CORRECT|84 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise MATCH THE COLUMN|26 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise Level 2|156 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 11)|6 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-CHEMICAL BONDING (ADVANCED)-Level 3
- In all expected compounds each central atom only uses its s and p-orbi...
Text Solution
|
- The comcept of redistribution of energy in different orbitals of an at...
Text Solution
|
- The comcept of redistribution of energy in different orbitals of an at...
Text Solution
|
- The comcept of redistribution of energy in different orbitals of an at...
Text Solution
|
- Drago suggested an emprical rule which is compatible with the energeti...
Text Solution
|
- Drago suggested an emprical rule which is compatible with the energeti...
Text Solution
|
- According to hybridisation theory, the % s-character in sp, sp^(2) and...
Text Solution
|
- According to hybridisation theory, the % s-character in sp, sp^(2) and...
Text Solution
|
- According to hybridisation theory, the % s-character in sp, sp^(2) and...
Text Solution
|
- PCl(5) is an example of a molecule having sp^(3)d-hybridisation. Three...
Text Solution
|
- PCl(5) is an example of a molecule having sp^(3)d-hybridisation. Three...
Text Solution
|
- PCl(5) is an example of a molecule having sp^(3)d-hybridisation. Three...
Text Solution
|
- The first compound of the noble gasees was made in 1962. Barlett and L...
Text Solution
|
- The first compound of the noble gases was made in 1962. Barlett and Lo...
Text Solution
|
- The first compound of the noble gasees was made in 1962. Barlett and L...
Text Solution
|
- According to MOT, two atomic orbitals overlap relsulting in the format...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
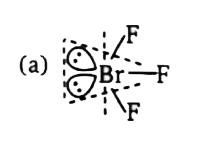 `implies ` Maximum number of atoms that can lie in a plane =4
`implies ` Maximum number of atoms that can lie in a plane =4 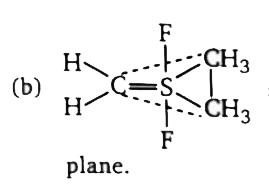 `implies` Hydrogen atoms bonded by `1s-sp^(2)` overlapping lie in axial
`implies` Hydrogen atoms bonded by `1s-sp^(2)` overlapping lie in axial 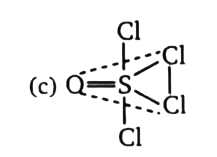 `impliesangleCl-S-Cl` is less than `120^(@) ` due to shifting of s-character in S=O bond. (d)
`impliesangleCl-S-Cl` is less than `120^(@) ` due to shifting of s-character in S=O bond. (d)