A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise ONE OR MORE ANSWER IS/ARE CORRECT|84 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise MATCH THE COLUMN|26 VideosCHEMICAL BONDING (ADVANCED)
VK JAISWAL|Exercise Level 2|156 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 11)|6 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-CHEMICAL BONDING (ADVANCED)-Level 3
- The first compound of the noble gasees was made in 1962. Barlett and L...
Text Solution
|
- According to MOT, two atomic orbitals overlap relsulting in the format...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- According to MOT, two atomic orbitals overlap resulting in the formati...
Text Solution
|
- Polar covalent molecules exhibit dipole moment. Dipole moment is equal...
Text Solution
|
- Polar covalent molecules exhibit dipole moment. Dipole moment is equal...
Text Solution
|
- Polar covalent molecules exhibit dipole moment. Dipole moment is equal...
Text Solution
|
- Polar covalent molecules exhibit dipole moment. Dipole moment is equal...
Text Solution
|
- Polar covalent molecules exhibit dipole moment. Dipole moment is equal...
Text Solution
|
- In general boiling point of covalent compounds is affect due to incre...
Text Solution
|
- In general boiling point of covalent compounds is affect due to incre...
Text Solution
|
- In general boiling point of covalent compounds is affect due to incre...
Text Solution
|
- The molecule in which an atom is associated with more than 8 electrons...
Text Solution
|
- The molecule in which an atom is associated with more than 8 electrons...
Text Solution
|
- The molecule in which an atom is associated with more than 8 electrons...
Text Solution
|
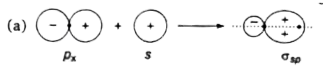 (No nodal plane between both nuclei)
(No nodal plane between both nuclei)