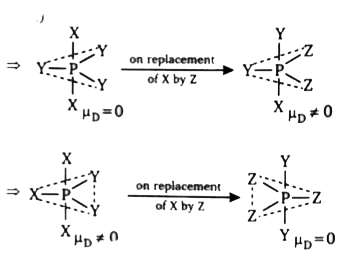
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL-CHEMICAL BONDING (ADVANCED)-ONE OR MORE ANSWER IS/ARE CORRECT
- Correct statement (s) about dipole moment of R(3)NO and R(3)PO is /are...
Text Solution
|
- In which of the following compounds observed bond angle is found to be...
Text Solution
|
- Two compounds PX(2)Y(3) and PX(3)Y(2) (Where P=phosphorous atom and X,...
Text Solution
|
- Corrrect order of bond angle in the given compounds is/are:
Text Solution
|
- The correct statement(s) is /are :
Text Solution
|
- Consider of following reactions CHF(3) overset(K(a))(to)CF(3)^(-)+H^...
Text Solution
|
- In which of the following molecules mu(exp) (observed dipole moment) i...
Text Solution
|
- Correct statement (s) regarding As(CH(3))F(2)Cl(2) molecule is/are:
Text Solution
|
- Which of the following species is/are having 'N-N' bond order =2?
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- Which of the following species is/are not know?
Text Solution
|
- Select correct order between following compounds:
Text Solution
|
- Which of the following is (are) V-shaped?
Text Solution
|
- Select correct order between given compounds .
Text Solution
|
- Which of the following equlibria would have highest and lowest value o...
Text Solution
|
- Which of the following process is/are assciated with change of hybridi...
Text Solution
|
- Which of the following are true?
Text Solution
|
- Which of the following statement is incorrect?
Text Solution
|
- Which of the following statements are not correct?
Text Solution
|
- In the structure of H(2)CSF(4), which of the following statement is/ar...
Text Solution
|