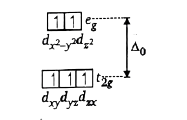Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
VK JAISWAL|Exercise LEVEL 1|202 VideosCO-ORDINATION COMPOUNDS
VK JAISWAL|Exercise LEVEL 3 (PASSAGE TYPE)|97 VideosCO-ORDINATION COMPOUNDS
VK JAISWAL|Exercise MATCH THE COLUMN|36 VideosCHEMICAL BONDING (BASIC)
VK JAISWAL|Exercise Level 3 (Passive 11)|6 Videosd-BLOCK ELEMENTS
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|6 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-CO-ORDINATION COMPOUNDS-SUBJECTIVE PROBLEMS
- How many pi-bonds are presennt in ferrocene?
Text Solution
|
- Consider the following complexes compounds (i) [Cu(NH(3))(4)][Cu(NO(...
Text Solution
|
- Consider the following carbonyl complex compounds. (i) Mo(CO)(x) (...
Text Solution
|
- If x and y are total number of electrons which are present in non-axia...
Text Solution
|
- Consider the following complex compounds: (i) [Pt(NH(3))(2)(SCN)(2)]...
Text Solution
|
- Consider the following transformation: Cr(CO)(x) to Cr(CO)(y)(NO)(z)...
Text Solution
|
- Brown colour of the complex [Fe(H(2)O)(5)(NO)]SO(4) is due to C. T. sp...
Text Solution
|
- Calculate |C.F.S.E| (mod value) is term of Dq. For complex ion [MnF(6)...
Text Solution
|
- Total number of geometrical isomers of [CoBrClI(CN)(H(2)O)(NH(3))]^(-)...
Text Solution
|
- What is CFSE of complex ion [FeF(6)]^(4-) in terms of Dq?
Text Solution
|
- How many more co-ordination isomers are possible of the compound [Cu(N...
Text Solution
|
- Total number of space (stereo) isomers of complex ion [Cr(gly)(en)(2)]...
Text Solution
|
- How many electrons are present in t(2g) set of d-orbitals of central m...
Text Solution
|
- A (Light pink colour complex) underset(Delta)overset(Pb(2)O//dil." "HN...
Text Solution
|
- Calculate value of "x+y" if x is the total number of sigma bonds and y...
Text Solution
|
- Consider the following complex compounds: (i) [Pt(NH(3))(2)(SCN)(2)]...
Text Solution
|
- Total number of complexes among the following which are opticaly activ...
Text Solution
|
- Consider the following complexes: (i) [FeIF(CN)(H(2)O)(en)] (ii) [...
Text Solution
|
- Consider the following ligands NH(2)^(-), acac, OH^(-), Gly, O(2)^(-),...
Text Solution
|
- M(CO)(x)(NO)(y) underset(-CO)overset(+NO) to M(NO)z Where EAN of me...
Text Solution
|