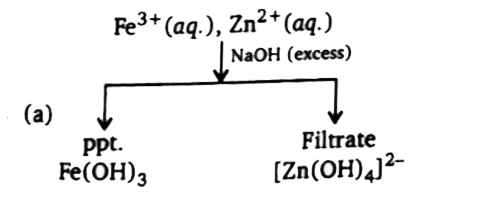
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL-TYPES OF REACTIONS-LEVEL 2
- Colour of CrO(4)^(2-)(aq.) is not changed by
Text Solution
|
- Mg(3)N(2)(s)+H(2)O overset(R.T.)to P darr+Q uarr Excess 'Q' gas does...
Text Solution
|
- Which of the following pair of cations cann be separated by excess NaO...
Text Solution
|
- Consider following reaction: Cl(2)(g)+H(2)O overset(R.T.)to P+Q If...
Text Solution
|
- Which of the following reagent can dissolves precipitate of HgSdarr
Text Solution
|
- Which of the following reaction is incorrect?
Text Solution
|
- Concentrated sodium hydroxiide can separate a mixture of:
Text Solution
|
- Select correct set of species which can't react with water but react w...
Text Solution
|
- Fe(Finely powdered)++HCl(dil.)toP+Quarr Compound 'P' does not precip...
Text Solution
|
- Which combination gives maximum number of products?
Text Solution
|
- Cu^(2+)(aq.) does not undergo redox reaction with solution:
Text Solution
|
- Hydrolysis of which of the following compound liberates acidic gas?
Text Solution
|
- The non-metal which does not react with water but reacts with alkali?
Text Solution
|
- A very dilute acidic solution of Cd^(2+)&Ni^(2+) gives only yelllwo pp...
Text Solution
|
- Thermal decomposition of which of the salt listed below yield a basic ...
Text Solution
|
- What are formed products, when aqueous solution of CuCl(2) and (NH(4))...
Text Solution
|
- Which of the following compound does not react with cold and dil. HNO(...
Text Solution
|
- The incorrect order of solubility in water is:
Text Solution
|
- The correct order of increasing solubility in water is:
Text Solution
|
- Bromine is commercially prepared from sea water by displacement reacti...
Text Solution
|