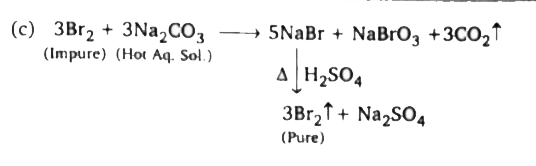A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL-TYPES OF REACTIONS-LEVEL 2
- The incorrect order of solubility in water is:
Text Solution
|
- The correct order of increasing solubility in water is:
Text Solution
|
- Bromine is commercially prepared from sea water by displacement reacti...
Text Solution
|
- Which of the following metal on burning in moist air does not give sme...
Text Solution
|
- Gas that can not be collected over water is:
Text Solution
|
- Compound having lowest thermal stability is:
Text Solution
|
- Which of the following statement is incorrect regarding Fe^(2+) and Fe...
Text Solution
|
- (NH(4))(2)Cr(2)O(7) on heating liberates a gas. The same gas will be ...
Text Solution
|
- Which of the following compound liberates acidic gas during its hydrol...
Text Solution
|
- Which of the following combination does not evolve Cl(2) gas?
Text Solution
|
- NH(3) gas does not liberate by which of the following combination?
Text Solution
|
- If salt Q undergoes redox reaction with H(2)S in acidic medium then wh...
Text Solution
|
- Metal sulphate (A) overset(Heat) to "oxide"(B)+gas(C)+gas(D)overset(Cr...
Text Solution
|
- Which of the following radical does not liberate gas with (Zn+dil. HCl...
Text Solution
|
- Which of the following cation does not give precipitate with H(2)S in ...
Text Solution
|
- NaCl(solid)+K(2)Cr(2)O(7)(solid)+conc.H(2)SO(4) overset(warm)toReddish...
Text Solution
|
- Diamagnetic gas neutral towards water is:
Text Solution
|
- Which of the following reagent can be used to separate AgCl and AgI?
Text Solution
|
- When PbO(2) reacts with conc. HNO(3) then evolved gas is:
Text Solution
|
- In a closed container there is a mixture of SO(2),CO(2) and O(2) gas,...
Text Solution
|