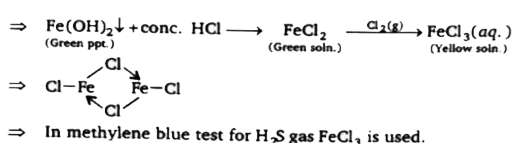A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL-TYPES OF REACTIONS-LEVEL 3
- Consider three P, Q, R, salts among them P and Q salts have different ...
Text Solution
|
- Consider three P, Q, R, salts among them P and Q salts have different ...
Text Solution
|
- Consider three P, Q, R, salts among them P and Q salts have different ...
Text Solution
|
- Three compound X, Y and Z were taken into three different laboratory v...
Text Solution
|
- Three compound X, Y and Z were taken into three different laboratory v...
Text Solution
|
- Three compound X, Y and Z were taken into three different laboratory v...
Text Solution
|
- In salts of polyatomic anion, as polarising power of cation increases,...
Text Solution
|
- In salts of polyatomic anion, as polarising power of cation increases,...
Text Solution
|
- Dioxygen directly reacts with nearly all metals annd non-metals except...
Text Solution
|
- Dioxygen directly reacts with nearly all metals annd non-metals except...
Text Solution
|
- Dioxygen directly reacts with nearly all metals annd non-metals except...
Text Solution
|
- Consider the following reactions and answer the following questions. ...
Text Solution
|
- Consider the following reactions and answer the following questions. ...
Text Solution
|
- Consider the following reactions and answer the following questions. ...
Text Solution
|
- Q. Compound 'X' is:
Text Solution
|
- Q. Incorrect statement 'Y' changes on heating:
Text Solution
|
- The unique behaviour of CU, having a positive E^(@) (reduction potenti...
Text Solution
|
- The unique behaviour of CU, having a positive E^(@) (reduction potenti...
Text Solution
|
- Q. When H(2)S gas was passed into filtrate (P), a coloured precipitate...
Text Solution
|
- Q. Precipitate (Q) was treated withdil. HCl and coloured solution was ...
Text Solution
|