A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL-ENVIRONMENTAL CHEMISTRY-ASSERTION-REASON TYPE QUESTIONS
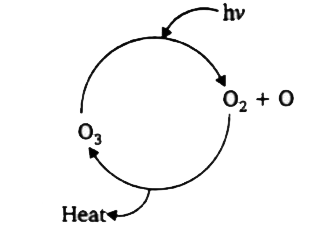
- When heathly earth's stratosphere contains a low concentration of ozon...
Text Solution
|
- Assertion (A) Green house effect was observed in houses used to grow p...
Text Solution
|
- Assertion: The pH of aicd rain is less than 5.6 Reason: Carbon dioxi...
Text Solution
|
- Assertion: Photochemical smog is oxidising in nature. Reason: Photoc...
Text Solution
|
- Assertion (A) : Carbon dioxide is one of the important greenhouse gase...
Text Solution
|
- Assertion (A) : Ozone is destroyed by solar radiation in upper streato...
Text Solution
|
- Assertion: Excessive use of chlorinated synthetic pesticides causes so...
Text Solution
|
- Assertion: If BOD level of water in a reservoir is less than 5 ppm it ...
Text Solution
|
- Assertion (A) Green house effect was observed in houses used to grow p...
Text Solution
|
- Assertion: The pH of aicd rain is less than 5.6 Reason: Carbon dioxi...
Text Solution
|
- Assertion: Photochemical smog is oxidising in nature. Reason: Photoc...
Text Solution
|
- Assertion (A) : Carbon dioxide is one of the important greenhouse gase...
Text Solution
|
- Assertion (A) : Ozone is destroyed by solar radiation in upper streato...
Text Solution
|
- Assertion: Excessive use of chlorinated synthetic pesticides causes so...
Text Solution
|
- Assertion: If BOD level of water in a reservoir is less than 5 ppm it ...
Text Solution
|