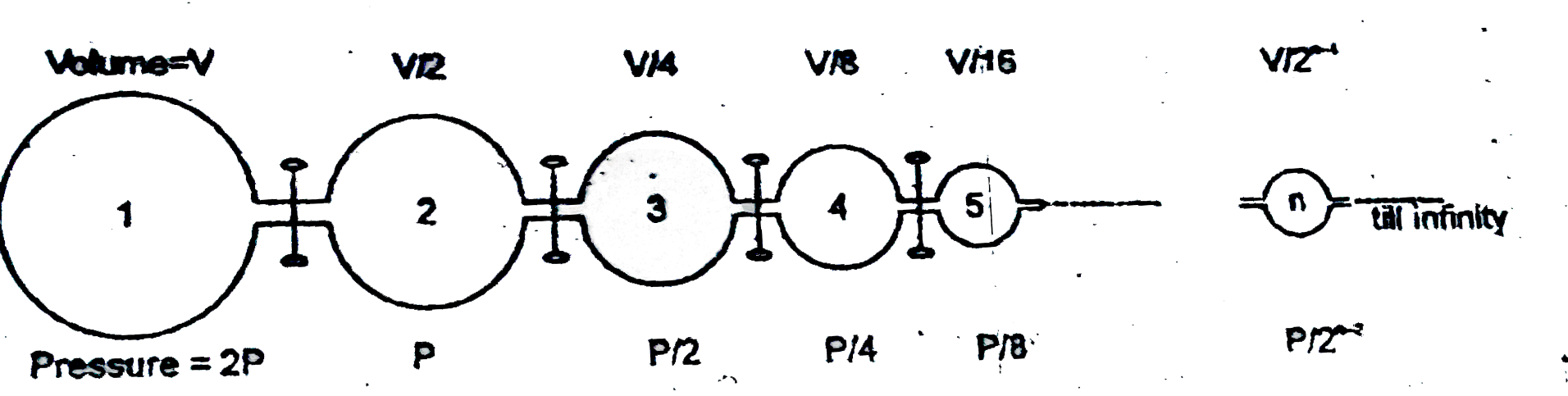Similar Questions
Explore conceptually related problems
Recommended Questions
- Inifinite number of flasks are connected to one another as shown above...
Text Solution
|
- A flask having a volume of 250.0 mL and containing air is heated to 10...
Text Solution
|
- Inifinite number of flasks are connected to one another as shown above...
Text Solution
|
- In the following figure, when the two stopcocks are opened, the total ...
Text Solution
|
- There are n connected having container having volume V, 2V,3V,...,nV s...
Text Solution
|
- Consider the arrangement of bulbs shown in the drawing. Each of three ...
Text Solution
|
- Two containers are connected by stopcock as shown. If initially P(O(2)...
Text Solution
|
- Consider three flasks in diagram below. Assuming that connecting tube ...
Text Solution
|
- Consider three flasks in diagram below. Assuming that connecting tube ...
Text Solution
|