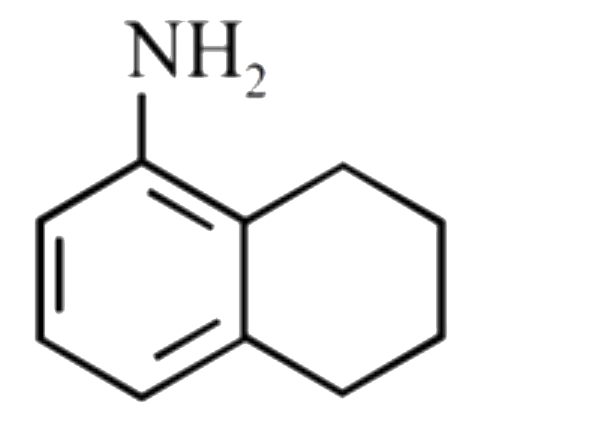
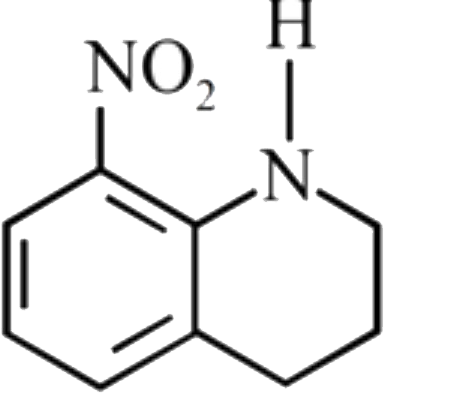
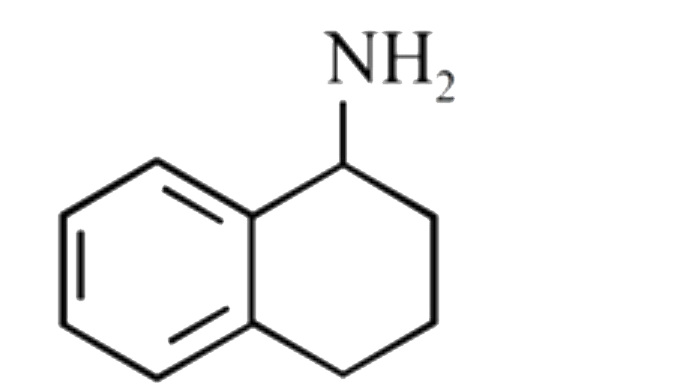
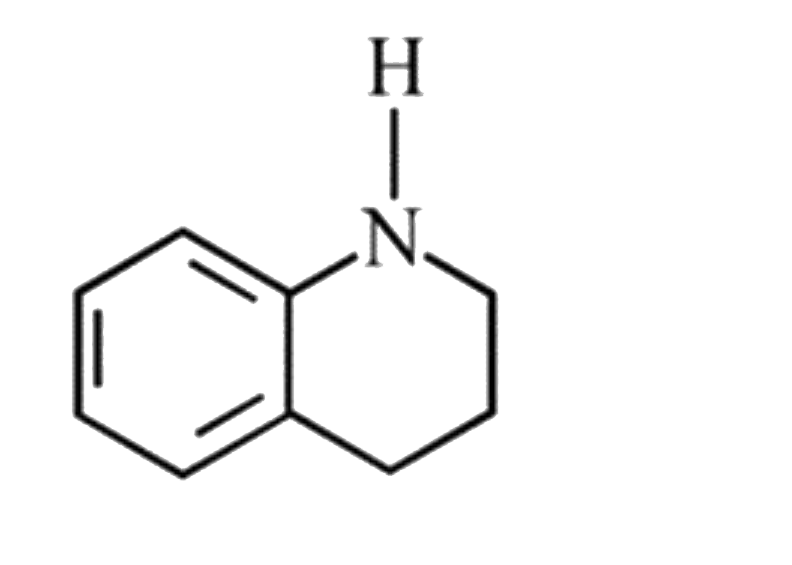
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 113-CHEMISTRY
- The vapour density of a volatile chloride of a metal is 95 and the spe...
Text Solution
|
- When potassium superoxide is dissolved in water, the products obtained...
Text Solution
|
- Which is the strongest base ?
Text Solution
|
- Two flasks A and B of equal volume containing equal masses of H2 and C...
Text Solution
|
- In the following sequence of reactions, the alkene affords the compoun...
Text Solution
|
- (H2N)(2)CO + X to N2 + CO2 + H2O Which nitrogen compound should X be...
Text Solution
|
- In fcc unit cell, atoms are numbered as shown below. The atoms not t...
Text Solution
|
- Which of the following is incorrect match?
Text Solution
|
- In the given reaction
Text Solution
|
- Which of the following gives yellow ppt. with H2PtCl6 ?
Text Solution
|
- 28 g of N(2) and 6 g of H(2) were mixed. At equilibrium 17 g NH(3) was...
Text Solution
|
- Compare bond lengths (x and y) for the following molecules
Text Solution
|
- What is the product of the reaction of methyl cyclohexene with BH3 - T...
Text Solution
|
- In Wilkinson's catalyst, the hybridization of central metal ion and it...
Text Solution
|
- CH3-underset(CH3)underset(|)(CH)-underset(CH3)underset(|)overset(CH3)o...
Text Solution
|
- Identify the correct statement regarding entropy
Text Solution
|
- Given that I(2)+2e^(-) rarr 2I^(c-)," "E^(c-)=0.54V Br(2)+2e^(...
Text Solution
|
- In P(4)O(10) molecule, bridging P - O bond length is
Text Solution
|
- A transition metal complex shows a magnetic moment of 5.9 B.M. at room...
Text Solution
|
- Acidified potassium permanganate is dropped over sodium peroxide taken...
Text Solution
|