A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 115-CHEMISTRY
- Which of the following pairs are enantiomers?
Text Solution
|
- The incorrect arrangement regarding the properties of the given specie...
Text Solution
|
- In which of these cyclic compounds you find the minimum angle strain?
Text Solution
|
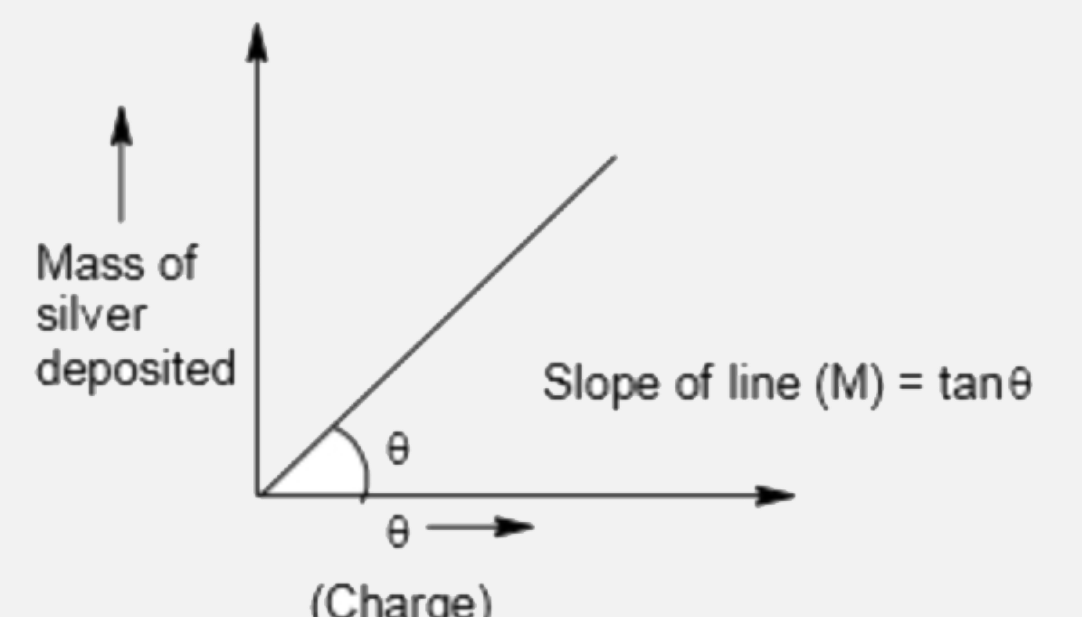
- Calculate the deposited mass of silver which is plotted against charge...
Text Solution
|
- Among the following , the paramagnetic compound is :
Text Solution
|
- For a monatomic gas, kinetic energy = E. The relation with rms velocit...
Text Solution
|
- Identify the correct hybridizations of atom 1 and 2, respectively, in ...
Text Solution
|
- For the preparation of a buffer of pH = 8.26, the amount of (NH4)(2)SO...
Text Solution
|
- Suppose the value of the equilibrium constant for the given reaction H...
Text Solution
|
- Positive iodoform test is given by
Text Solution
|
- The correct order of polarizability for I^(-),Br^(-),CI^(-),F^(-) is :
Text Solution
|
- In the reaction PCl(3) + Cl(2) to PCl5, which of the following is corr...
Text Solution
|
- Which of the following products is formed when n - heptane is passed o...
Text Solution
|
- Which one of the following is an inner orbital complex as well as diam...
Text Solution
|
- The correct order of mobility of alkali metal ions in aqueous solution...
Text Solution
|
- Calculate spin only magnetic moment of Fe^(3+) ion.
Text Solution
|
- An organic compound contains 49.3% carbon. 6.84% hydrogen and its vapo...
Text Solution
|
- The noble gas with the highest boiling point is :
Text Solution
|
- If pKa = 4 and ka = Ca^2, then find van't Hoff factor for a weak HA ty...
Text Solution
|
- What will be normality of the resultant solution when 500 ml 1 N H2SO4...
Text Solution
|