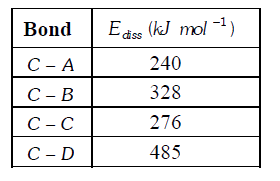A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The table shown below gives the bond dissociation energies (E("diss"))...
Text Solution
|
- The table given below lists the bound dissociation enegrgy (E(diss)) f...
Text Solution
|
- Which element-element bond has the highest bond dissociation energy ?
Text Solution
|
- The table given below lists the bond dissociation energy (E("diss")) f...
Text Solution
|
- The table shown below gives the bond dissociation emergies (E("diss"))...
Text Solution
|
- दो तत्वों A तथा B के परमाणुओं की बंधन-उर्जाये क्रमश: E(a) तथा E(b) है!...
Text Solution
|
- निचे दर्शायी गयी सारणी कार्बन तथा तत्त्वों A,B,C तथा D के परमाणुओं ...
Text Solution
|
- The table shown below gives the bond dissociation energies (E("diss"))...
Text Solution
|
- The H-H bond dissociation enthalpy of H2 is , is the highest for a sin...
Text Solution
|