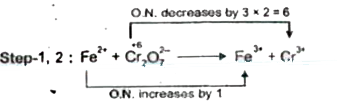
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- For the redox reaction xFe^(2+)+yCr(2)O(7)^(2-)+zH^(+) rarr Fe^(3+)+...
Text Solution
|
- For the redox reaction xFe^(2+)+yCr(2)O(7)^(2-)+zH^(+) rarr Fe^(3+)+...
Text Solution
|
- For the redox reaction Cr(2)O(7)^(-2)+H^(+)+Ni rarr Cr^(3)+Ni^(2+)+H(2...
Text Solution
|
- In the following unbalanced redox reaction, Cu(3)P+Cr(2)O(7)^(2-) ra...
Text Solution
|
- Consider the following reaction : xMnO(4)^(-)+yC(2)O(4)^(2-)+zH^(+) to...
Text Solution
|
- निम्नलिखित अभिक्रिया पर विचार कीजिये: xMnO(4)^(-)+yC(2)O(4)^(2-)+zH...
Text Solution
|
- The coefficients w, x,y,z in the reaction w Cr(2)O(7)^(2-) + xFe^(2+) ...
Text Solution
|
- Consider the following reaction: xMnO(4)^(-)+yC(2)O(4)^(2-)+zH^(+) t...
Text Solution
|
- निम्नलिखित रेडॉक्स अभिक्रिया को आयन - इलेक्ट्रॉन विधि द्वारा संतुलित क...
Text Solution
|