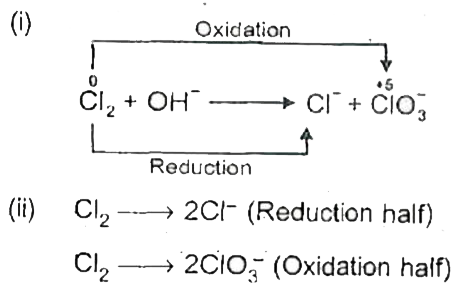
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- In passing chlorine gas through a concentrated solution of alkali we g...
Text Solution
|
- Write the balanced equation for the following chemical reaction: "Hy...
Text Solution
|
- Write balanced chemical equations for the following reactions : (a) ...
Text Solution
|
- In passing chlorine gas through a concentrated solution of alkali we g...
Text Solution
|
- In passing chlorine gas through a concentrated solution of alkali we g...
Text Solution
|
- In passing chlorine gas through a concentrated solution of alkali we g...
Text Solution
|
- Write with balanced chemical equation, what happens when chlorine gas ...
Text Solution
|
- Write with balanced chemical equation, what happens when chlorine gas ...
Text Solution
|
- When dry chlorine gas is passed through silver chlorate heated to 90^@...
Text Solution
|