A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
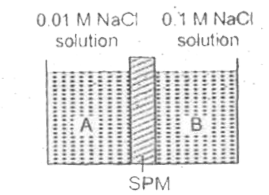
- Two solutions marked as A and B are separated through semipermeable me...
Text Solution
|
- $ Both the solute and solvent can pass through a semipermeable membran...
Text Solution
|
- Two solutions A and B are separated by semipermeable membrane. If liqu...
Text Solution
|
- Two solutions A and B are separated by semipermeable membrane. If liqu...
Text Solution
|
- Two solutions A and B are separated by semipermeable membrane. If liqu...
Text Solution
|
- In the phenomenon of osmosis through the semipermeable membrane
Text Solution
|
- Two solution marked as A and B are seprated through semipermeable memb...
Text Solution
|
- In the phenomenon of osmosis through the semipermeable membrane
Text Solution
|
- Two solutions marked as A and B are separated through semipermeable me...
Text Solution
|