A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
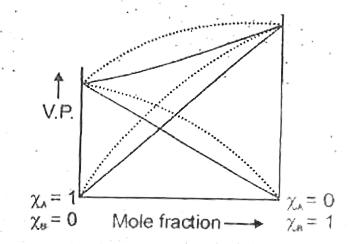
- Vapour phase difference for a solution is given below if dotted line r...
Text Solution
|
- For an ideal binary liquid solution with P(A)^@gtP(B)^@x(A) and y(A) r...
Text Solution
|
- धनात्मक विचलन वाले विलयन व् ऋणात्मक विचलन वाले विलयन में पाँच अंतर लिख...
Text Solution
|
- धनात्मक विचलन वाले विलयन व ऋणात्मक विचलन वाले विलयन में पाँच अंतर लिखि...
Text Solution
|
- Vapour phase diagram for a solution is given below if doted line repre...
Text Solution
|
- Vapour phase difference for a solution is given below if dotted line r...
Text Solution
|
- धनात्मक विचलन वाले विलयन व ऋणात्मक विचलन वाले विलयन में तीन अंतर लिखिए...
Text Solution
|
- When an ideal binary solution is in equilibrium with its vapour, molar...
Text Solution
|
- When an ideal binary solution is in equilibrium with its vapour, molar...
Text Solution
|