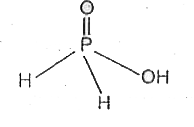
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- How do you account for reducing behaviour of H(3)PO(2) on the basis of...
Text Solution
|
- Strong reducing behaviour of H(3)PO(2) is due to:
Text Solution
|
- How is the reduction ability of H(3)PO(2) and H(3)PO(3) accounted on t...
Text Solution
|
- How do you account for reducing behaviour of H(3)PO(2) on the basis of...
Text Solution
|
- How do you account for the reducing behaviour of H(3)PO(2) ? Or (i) ...
Text Solution
|
- How do you account for the reducing behaviour of H(3)PO(2) ? Or (i) Dr...
Text Solution
|
- Strong reducing behaviour of H(3)PO(2) is due to
Text Solution
|
- How do you account of the reducing behaviour for H(3)PO(2) ?
Text Solution
|
- How do you account for the reducing behaviour of H(3)PO(2) on the basi...
Text Solution
|