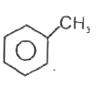
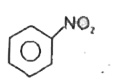
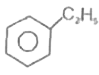
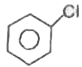
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTON - A|45 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION - B|25 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise Try Yourself|40 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
AAKASH INSTITUTE|Exercise Try Yourself|33 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - D) (Assertion -Reason Type Question)|24 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-HALOALKANES AND HALOARENES -EXERCISE
- Which of the following will react most readily with HI ?
Text Solution
|
- The isomer of C(6)H(14) which will give maximum number of monochlorode...
Text Solution
|
- Which of the following is most reactive towards electrophillic aromat...
Text Solution
|
- Amongst the C-X bond the correct bond energy order is
Text Solution
|
- Which of the following has highest boilling point ?
Text Solution
|
- Out of the following compounds which one will have zero dipole moment ...
Text Solution
|
- Treatment of ammonia with excess of ethyl chloride will yield
Text Solution
|
- Identity Z in the following sequence
Text Solution
|
- Most reactive halide towards S(N^(1)) reactions is
Text Solution
|
- Which of the following is most reactive toward nucleophilic substituti...
Text Solution
|
- NBS is a specific reagent for
Text Solution
|
- In reaction C(2)H(5)OH+Hxoverset(ZnX(2))rarrC(2)H(5)X+H(2)O the order ...
Text Solution
|
- Which of the following is not true for S(N^(1)) reaction ?
Text Solution
|
- 2-Chloro-2-methylpropane on reaction with aqueous KOH gives X as the m...
Text Solution
|
- Which of the following does not form Grignard reagent on reaction with...
Text Solution
|
- 1-phenyl-2-chloropropane on treating with alc. KOH gives mainly
Text Solution
|
- An alkyl halide on reaction with sodium in the presence of ether gives...
Text Solution
|
- Ethyl bromide reacts with lead sodium alloy to form:
Text Solution
|
- Allybromide on dehydrobromination gives
Text Solution
|
- Identify the major product
Text Solution
|