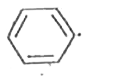
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION - B|25 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION - C|38 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise EXERCISE|40 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
AAKASH INSTITUTE|Exercise Try Yourself|33 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - D) (Assertion -Reason Type Question)|24 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-HALOALKANES AND HALOARENES -ASSIGNMENT SECTON - A
- Product (P) is
Text Solution
|
- Complete the following reaction
Text Solution
|
- Which is most stable radical ?
Text Solution
|
- The reagent R is
Text Solution
|
- Which of the following halogen exchange reaction will occur in ac...
Text Solution
|
- Complete the following reaction
Text Solution
|
- C(6)H(5)CH(3) overset(Br(2)//FeBr(3))(to) the reaction is called
Text Solution
|
- C(6) H(5)N2^+Cl^-overset(Cu(2)Cl(2)//HCl)(rarr) , this reaction is nam...
Text Solution
|
- which is more reactive nucleophile in polar protic solvent ?
Text Solution
|
- Which is more reactive nucleophile in polar aprotic solvent ?
Text Solution
|
- Which can not act as an ambident nucleophile ?
Text Solution
|
- Which of the following solvent is suitable for S(N)1 reaction ?
Text Solution
|
- For S(N)1 mechanism which of the following is correct ?
Text Solution
|
- The reaction , CH(3)Br +OH^(-)rarrCH(3)OH+Br^(-) obeys the mechanism
Text Solution
|
- Product P (major) is
Text Solution
|
- CH(3) - underset(CH(3))underset(|)overset(CH(3))overset(|)(C ) - Br un...
Text Solution
|
- As S(N^(2)) reaction at an asymmetric carbon of a compound always give...
Text Solution
|
- overset("SOCl"(2))(rarr) (A) overset("KCN")(rarr) (B) overset(H^(+))(r...
Text Solution
|
- Which one is the most reactive towards S(N)1 reaction ?
Text Solution
|
- The reaction goes through
Text Solution
|