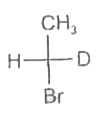
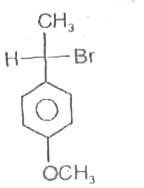
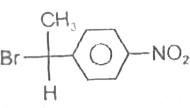
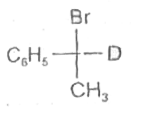
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION - C|38 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTION -D|15 VideosHALOALKANES AND HALOARENES
AAKASH INSTITUTE|Exercise ASSIGNMENT SECTON - A|45 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
AAKASH INSTITUTE|Exercise Try Yourself|33 VideosHYDROCARBONS
AAKASH INSTITUTE|Exercise Assignment(Section - D) (Assertion -Reason Type Question)|24 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-HALOALKANES AND HALOARENES -ASSIGNMENT SECTION - B
- Which one of the following is a free - radical substitution reaction ?
Text Solution
|
- Which of the following compounds undergoes mucleophilic substitution r...
Text Solution
|
- Replacement of CI of chlorobenzene to give pheno1 require drastic cond...
Text Solution
|
- Chloropicrin is obtained by the reaction of
Text Solution
|
- The raction of toluene with CI(2) in presence of FeCI(3) gives X and r...
Text Solution
|
- What is B ?
Text Solution
|
- Ethylidene chloride on treatement with aq. KOH gives
Text Solution
|
- The correct order of reactivity of the following compounds towards aqu...
Text Solution
|
- Which among the following is the correct order of melting point ?
Text Solution
|
- For which of the following G, above reaction will be the fastest ?
Text Solution
|
- Which of the following is least reactive towards nucleophilic substitu...
Text Solution
|
- Among the following The correct order of reactivity towards ArS...
Text Solution
|
- Which of the following does not give yellow precipitate with "AgNO"(3)...
Text Solution
|
- What is C ?
Text Solution
|
- The product (C ) is
Text Solution
|
- In the given reaction The product will be
Text Solution
|
- udner identical conditions, solvolysis of which of the following subst...
Text Solution
|
- The correct order of reactivity towards S(N)1 reaction is
Text Solution
|
- When cis-but-2-ene is treated with Br(2) in "CCl"(4) medium the produc...
Text Solution
|
- CH3-Cl+NaI overset("Acetone")iff CH3-I+NACl Above equilibrium is mor...
Text Solution
|