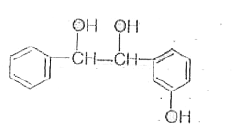
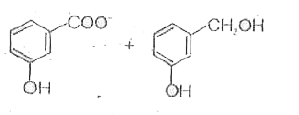
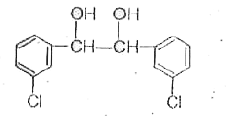
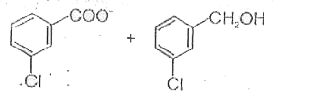A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- When m-chlorobenzaldehyde is treated with 50% Ba(OH)(2) solution, the ...
Text Solution
|
- pH of saturated solution of Ba(OH)(2) is 12. The value of solubility p...
Text Solution
|
- Which m-pchlorobenzaldehyde is treated with 50% KOH solution, the prod...
Text Solution
|
- The pH of 0.001 M Ba(OH)(2) solution will be :-
Text Solution
|
- pH of saturated solution of Ba(OH)(2) is 12. The value of solubility p...
Text Solution
|
- The pH of 0.001 M Ba(OH)(2) solution will be
Text Solution
|
- The pH of 0.05 M Ba (OH)(2) solution is
Text Solution
|
- When m-chlorobenzaldehyde is treated with 50% KOH solution, the produc...
Text Solution
|
- जब m -क्लोरोबेन्जल्डिहाइड की क्रिया 50% KOH विलयन से कराते हैं तो प्रा...
Text Solution
|