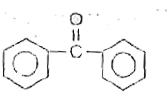
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Iodoform reaction is given by
Text Solution
|
- At higher temperature, iodoform reaction is given by the dilute soluti...
Text Solution
|
- Explain iodoform reaction.
Text Solution
|
- At higher temperature, iodoform reaction is given by:
Text Solution
|
- At higher temperature, iodoform reaction is given by
Text Solution
|
- Iodoform reaction is shown by
Text Solution
|
- Which one of the following compounds will not given iodoform by the re...
Text Solution
|
- Which one of the following compounds will not given iodoform by the re...
Text Solution
|
- Iodoform reaction is given by
Text Solution
|