Similar Questions
Explore conceptually related problems
Recommended Questions
- For a reaction ,(d[X])/(d t) is ewqual to
Text Solution
|
- The rate of reaction is expressed as : (1)/(2)(+d)/(d t)[C] = (1)/(3)(...
Text Solution
|
- A + B rarr Product, (d x)/(d t) = k[A]^(a)[B]^(b) If ((d x)/(d t)) = k...
Text Solution
|
- For a reaction ,(d[X])/(d t) is ewqual to
Text Solution
|
- (d^(s+t-:d^(s))-:d^(t) का मान ज्ञात करें।
Text Solution
|
- Which among the following plots are linear (a -x) is the concentration...
Text Solution
|
- If a^(x) = b^(y) = c^(z) = d^(t) and a ,b ,c d are in G.P . Then x, y,...
Text Solution
|
- डी. डी. टी. है एक-
Text Solution
|
- If x = (t^(3))/(3) - (5)/(2)t^(2) + 6t + 1, then the value (d^(2)x)/(d...
Text Solution
|
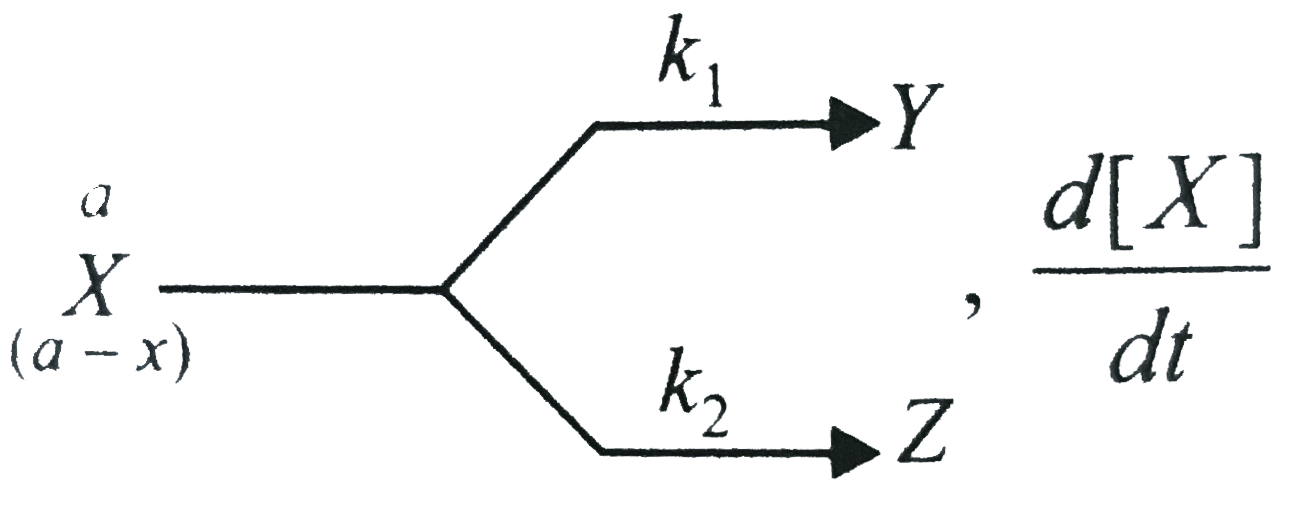 ,`(d[X])/(d t)` is ewqual to
,`(d[X])/(d t)` is ewqual to