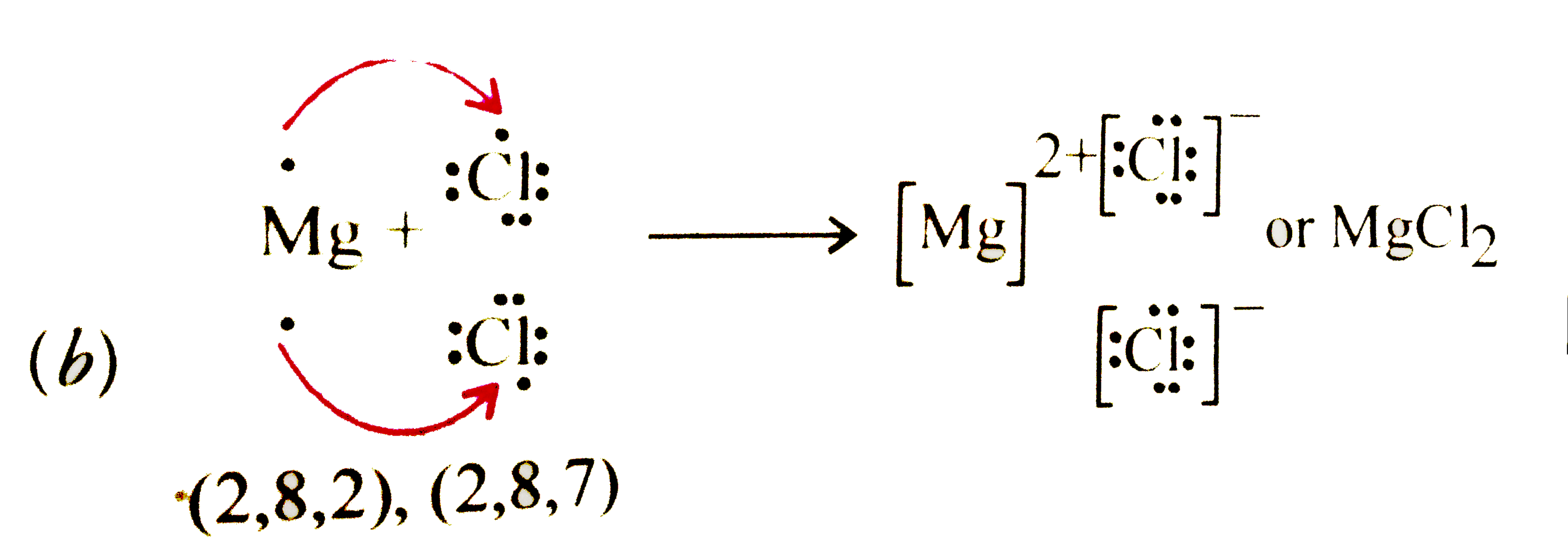Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON-METALS
DINESH PUBLICATION|Exercise Long Answer Questions|23 VideosMETALS AND NON-METALS
DINESH PUBLICATION|Exercise HOTS|7 VideosMETALS AND NON-METALS
DINESH PUBLICATION|Exercise Very Short Answer Questions|67 VideosCHEMICAL REACTIONS AND EQUATIONS
DINESH PUBLICATION|Exercise Multiple Choice Questions|18 VideosPERIODIC CLASSIFICATION OF ELEMENTS
DINESH PUBLICATION|Exercise PBQs|28 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-METALS AND NON-METALS-Short Answer Questions
- Give reason for the following (i) Iron grills are frequently painte...
Text Solution
|
- In one method of rust prevention, the iron is not coated with anything...
Text Solution
|
- Compound X and aluminium are used to join railway tracks. (a) Idemjfy ...
Text Solution
|
- An ore on treatment with dilute hydrochloric acid produces brisk effer...
Text Solution
|
- State the property utilized in the following : (i) Graphite in maki...
Text Solution
|
- (i) Carbonate and sulphide ores are usually converted into oxides duri...
Text Solution
|
- The reaction of metal 'X' with Fe(2)O(3) is highly exothermic and is u...
Text Solution
|
- Aluminium oxide and zinc oxide react with both acids are bases to prod...
Text Solution
|
- Describe an activity to show that metals are good conductors of electr...
Text Solution
|
- (a) In the electrorefining of impure copper metal, what are used as ca...
Text Solution
|
- (a) What is an alloy ? How is it prepared ? Give two examples of alloy...
Text Solution
|
- Write one example each of the following metals : (a) Most malleable...
Text Solution
|
- A metal 'P' when exposed to moist air for longer period of times, lose...
Text Solution
|
- Name the constituents elements of the alloys (i) Brass (ii) Bronze (ii...
Text Solution
|
- Hydrogen gas is not evolved when most metals react with nitric acid. S...
Text Solution
|
- (a) Why are metals not generally found in their free state ? (b) If...
Text Solution
|
- Write balanced chemical equation for the reactions taking place when ...
Text Solution
|
- An element 'X' burns in oxygen to form an electrovalent compound XO. S...
Text Solution
|
- A metal E is stored under kerosene. When a small piece of it is left o...
Text Solution
|
- Write the names and symbols of two most reactive metals. Explain by dr...
Text Solution
|