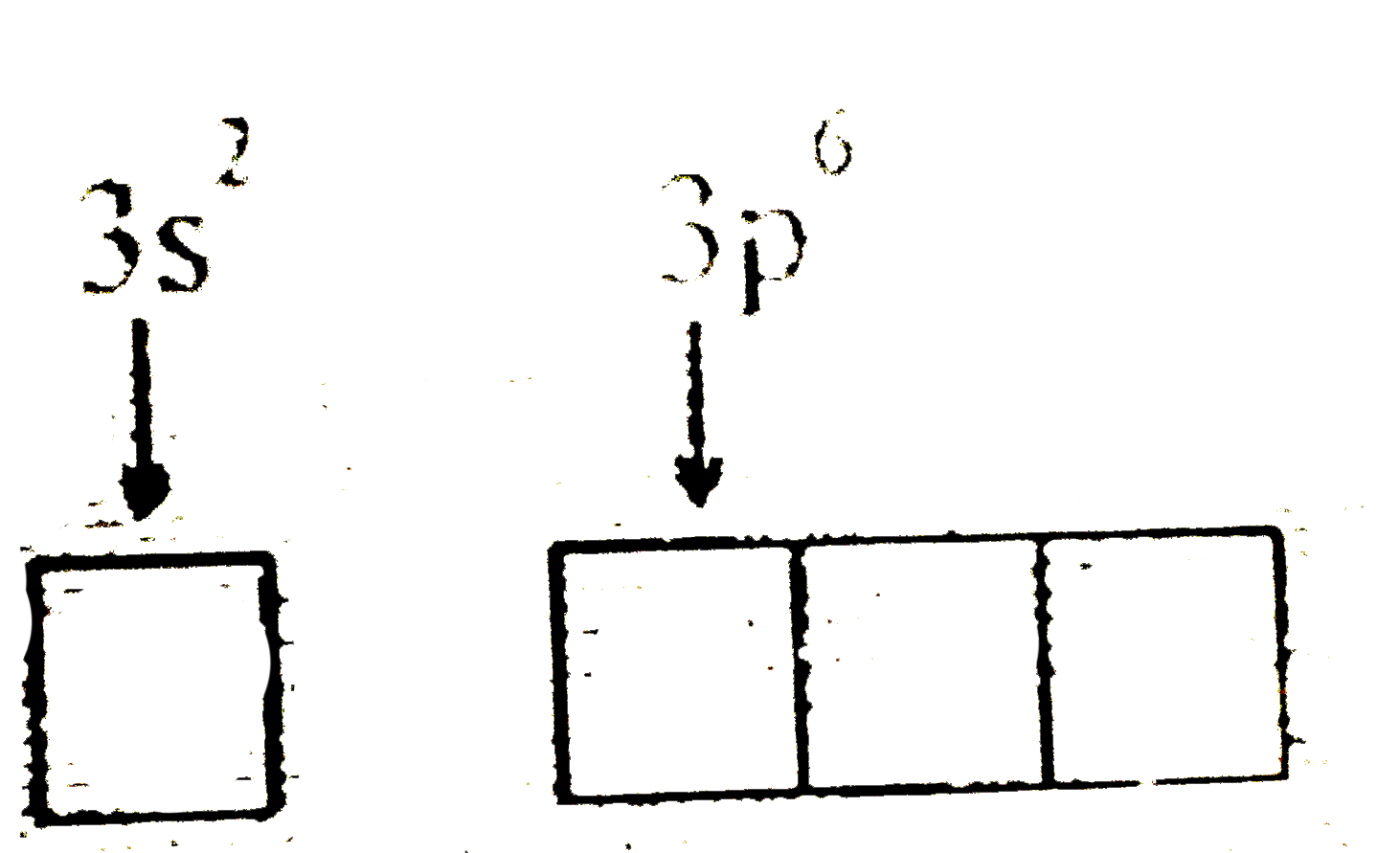Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-TEST PAPERS-PHYSICS
- What is maximum number of electron in an atom whose highest energy ele...
Text Solution
|
- Find minimum value of atomic number for which all orbitals of n=3 are ...
Text Solution
|
- If spin quantum number has 4 possible values. S=+1,-1,+(1)/(2),-(1)/...
Text Solution
|
- How many set of 4 quantum numbers are possible for last electron of Sc...
Text Solution
|
- A particle is projected wityh velocity 20 ms^(-1) at angle 60^(@) abov...
Text Solution
|
- A swimmer can swim with 5ms^(-1) in still water. He wants to cross a r...
Text Solution
|
- Figure shown V(y)-t graph of a particle projected from the ground. The...
Text Solution
|
- Block M slide donw on frictionless inclined plane as shown. Find frict...
Text Solution
|
- Two particles are projected from two points 4m apart on the horizontal...
Text Solution
|
- A block is thrown with 14ms^(-1) over a long stationary plank as shown...
Text Solution
|
- A 60kg man is running on horizontal road with acceleration 2ms^(-1) fr...
Text Solution
|
- In a uniform circular motion, choose correct statement(s):
Text Solution
|
- A particle is doing uniform circular motion as shown. Magnitude of ave...
Text Solution
|
- A stone is dropped from a building, and 2 seconds later another stone ...
Text Solution
|
- A sphere is placed on two similar cubes as shown. All surfaces are smo...
Text Solution
|
- Two masses are joined together by a massless, inextensible string. A v...
Text Solution
|
- You read about a car accidnet in the newspaper. A car travelling at 70...
Text Solution
|
- You step into an elevator, and it accelerates to a downward speed of 9...
Text Solution
|
- Blocks of 1,0,2,0 and 3.0 kg are lined up on a table, as shown in fig....
Text Solution
|
- Determine the work done by the frictional force in moving a block of m...
Text Solution
|