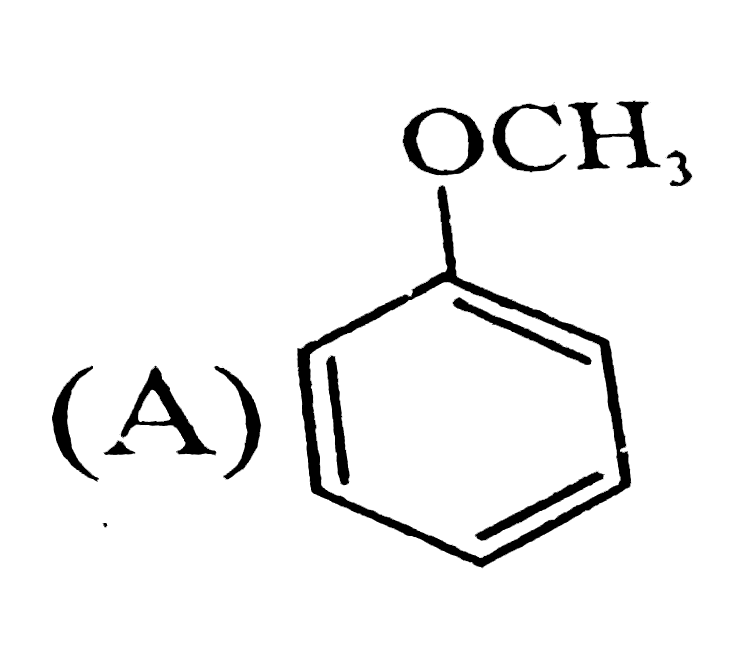
A
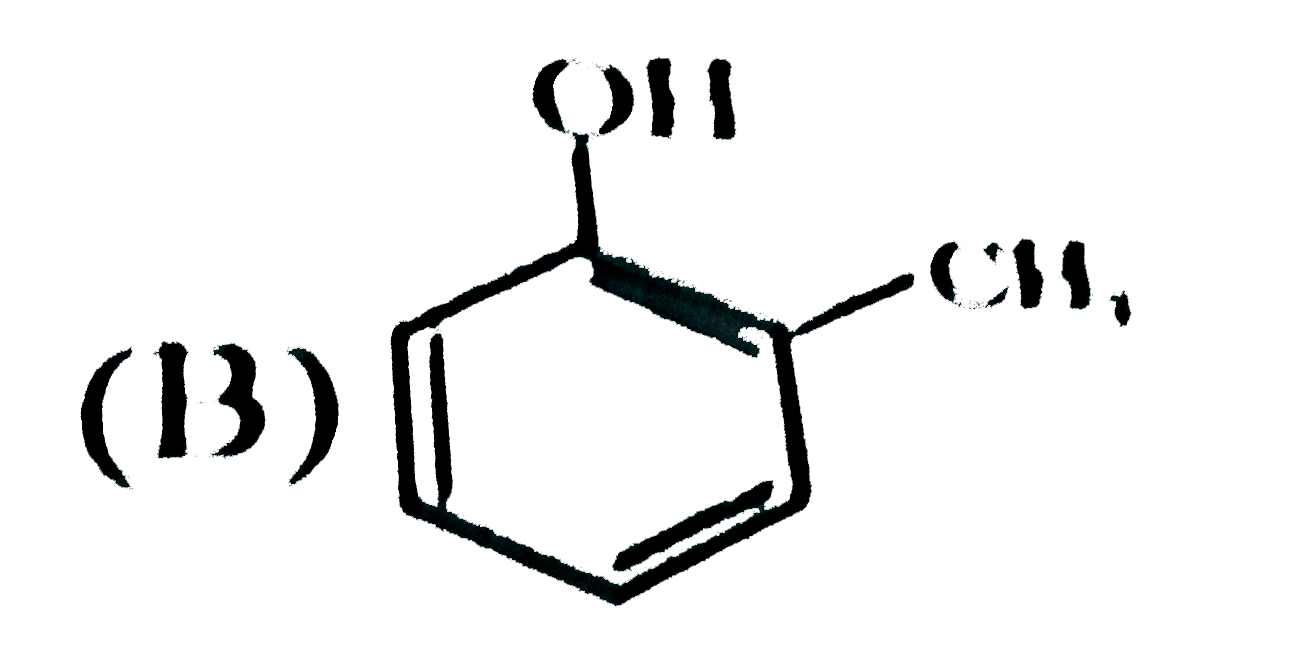
B
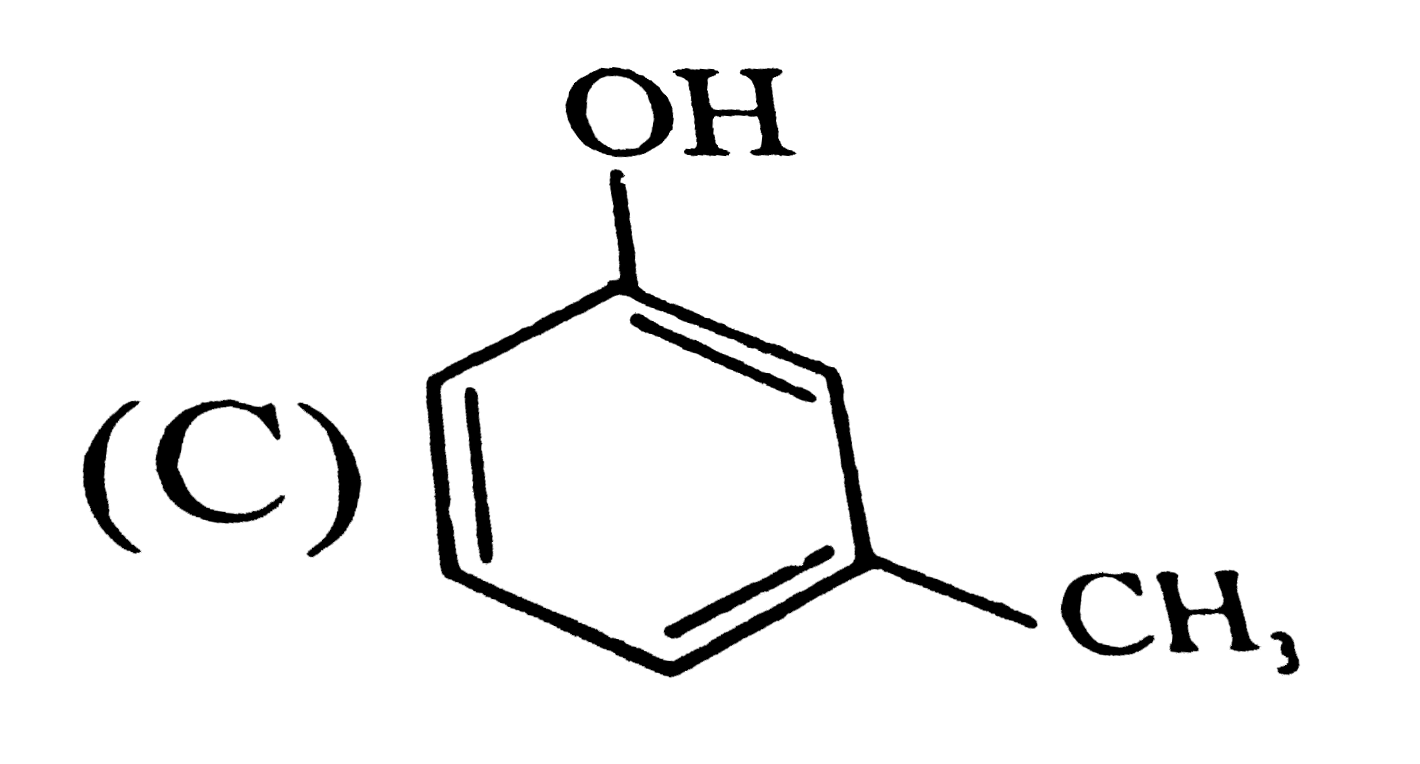
C
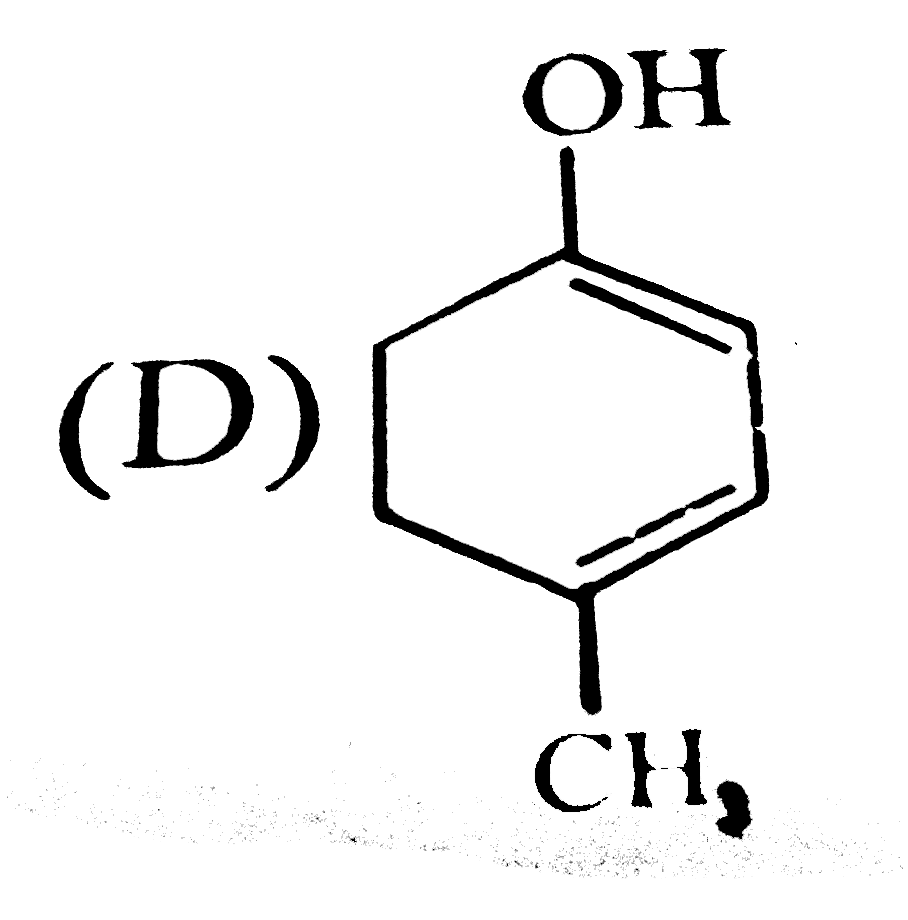
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Compound A (C(7) H(8) O) is insoluble in water, dilute HCl & aqueous N...
Text Solution
|
- Compound 'P', C(7)H(8)O is insolution in water , dilute when HCI and N...
Text Solution
|
- Compound A,C(7)H(8)O , is insoluble in water, dilute HCl , and aquenou...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound A (C(7)H(8)O) is insoluble in water , dilute HCL and aqueous ...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- Compound (A)C(7)H(8)O is insoluble in aqueous NaHCO(3) and dissolves i...
Text Solution
|
- A compound 'A' (C(7) H(8) O) is insoluble in water, dilute HCl and ...
Text Solution
|