Similar Questions
Explore conceptually related problems
Recommended Questions
- A cell diagram shown below contains of one litre of buffer solution of...
Text Solution
|
- Two litre solution of a buffer mixture containing 1.0 M NaH(2)PO(4) an...
Text Solution
|
- pH when solution containing HA (K(a)=10^(-6)) and NaA show maximum buf...
Text Solution
|
- In a concentration cell the same reagents are present in both the anod...
Text Solution
|
- A cell diagram shown below contains of one litre of buffer solution of...
Text Solution
|
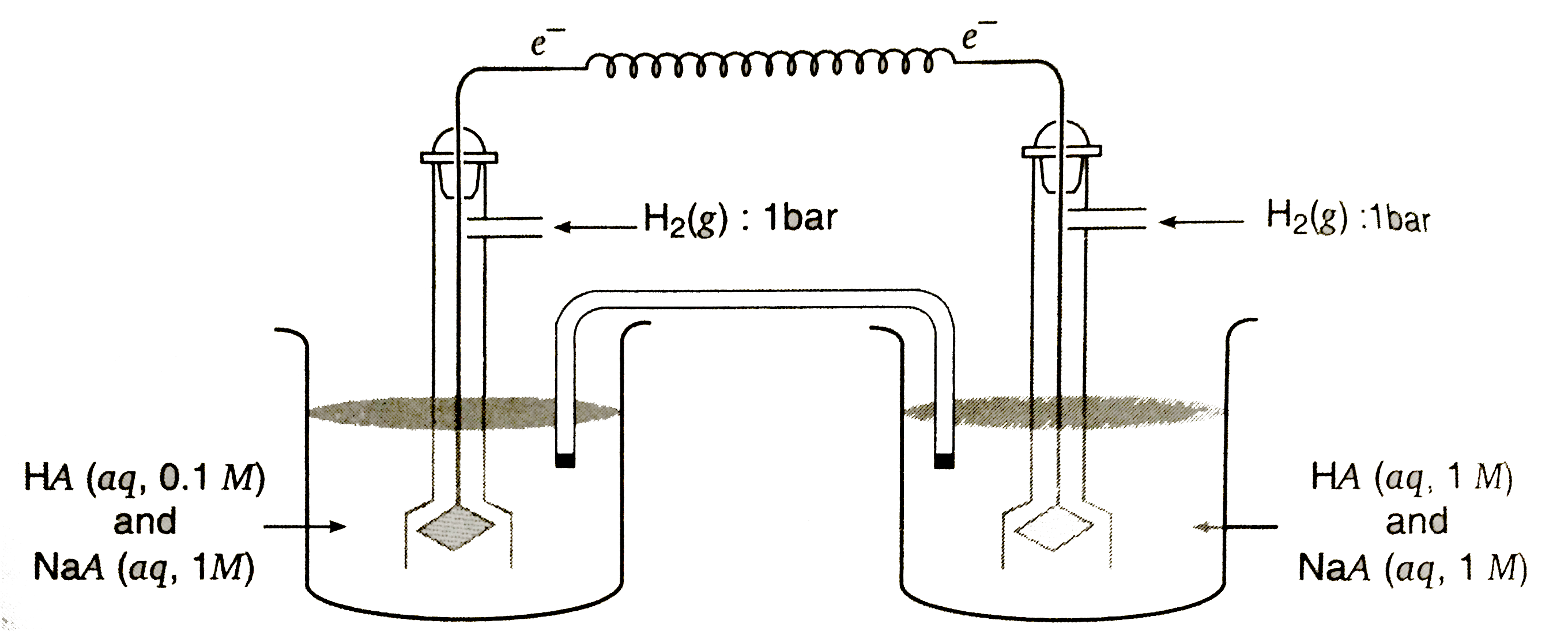
- In the concentration cell Pt (H(2)) |(HA (0.1M)),(Na A (1M))||(HA (1...
Text Solution
|
- The middle compartment of the hydrogen-oxygen fuel cell contains, hot ...
Text Solution
|
- Assertion : A solution containing acetic acid and sodium acetate acts ...
Text Solution
|
- For a weak acid, HA pKa=5. What is the effective range of pH for a buf...
Text Solution
|