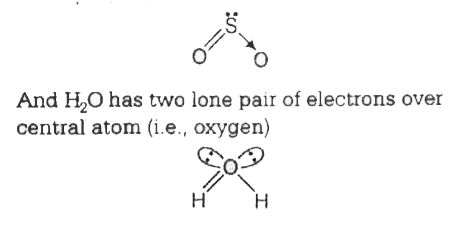A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-AP EAMCET ENGINEERING ENTRANCE EXAM QUESTION PAPER 2017 (SOLVED)-Chemistry
- The work functions of Ag, Mg, K and Na respectively in eV are 4.3, 3.7...
Text Solution
|
- The increasing order of acidity of the following carboxylic acids is
Text Solution
|
- Colloidal solution of gold is in different colours like red, purple, b...
Text Solution
|
- The Lewis structure for O(3) molecule is given below. The correct fo...
Text Solution
|
- Identify the statemements which are not correct form the following a...
Text Solution
|
- The enthalpy of formation (DeltarH) of methanol, formaldehyde and wate...
Text Solution
|
- Copper matte contains
Text Solution
|
- At 27^@C ina 10L flask 4.0g of an ideal gaseous mixture conatining He ...
Text Solution
|
- S + conc. H2SO4 rightarrow X+Y here X is a gas and Y is a liquid and b...
Text Solution
|
- Identify the correct statements from the following Electromeric effec...
Text Solution
|
- Which of the following is used in the estimation of carbon monoxide?
Text Solution
|
- Which one of the following statements is not correct?
Text Solution
|
- The drug, which was designed to prevent the interactions of histamine ...
Text Solution
|
- Identify the correct statements for a ring system to exhibit aromatici...
Text Solution
|
- Two oxides of a non-metal X contain 50% and 40% of non -metal respecti...
Text Solution
|
- An element has a body centered cubic structure with a unit cell edge l...
Text Solution
|
- 20% of a first order reaction was found to be completed at 10 a.m at...
Text Solution
|
- The gaseous products formed at cathode (X) and anode (Y), when an aque...
Text Solution
|
- How many mililiters of 20 volume of H2O2 solution is needed to react c...
Text Solution
|
- Same amount of electricity is passed through aqueous solutions of AgNO...
Text Solution
|