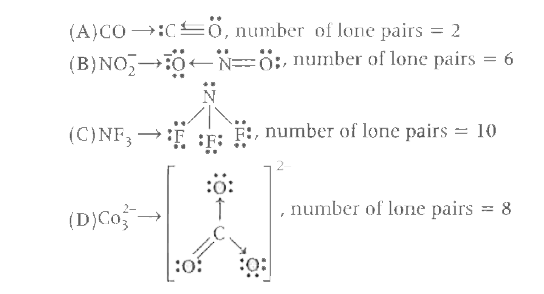
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Arrange the following species in the increasing order of long pairs of...
Text Solution
|
- Arrange the following compounds in the order of decreasing molar condu...
Text Solution
|
- Which of the following species have same bond order and same shape (a)...
Text Solution
|
- Among the following species, identify the isostuctural pairs NF(3). NO...
Text Solution
|
- Arrange the following compounds in order of increasing molar conductiv...
Text Solution
|
- Among the following species, identify the isostuctural pairs NF(3). ...
Text Solution
|
- Arrange the following compounds in order of increasing molar conductiv...
Text Solution
|
- Arrange the following in increasing order of acidity: NO(2),Al(2)O(3),...
Text Solution
|
- Arrange the following species in increasing order of acidic strength :...
Text Solution
|