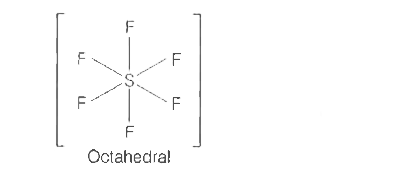
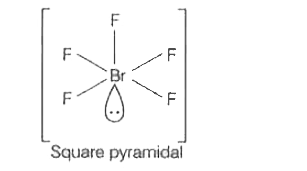
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-AP EAMCET SOLVED PAPER 2019-CHEMISTRY
- The energies of an electron in first orbit He ^(+) and in third orbit...
Text Solution
|
- How many orbitals is/are possible with n=3 l-1 and m(1) = -1 value ?
Text Solution
|
- If four elements with atomic numbers Z-2, Z-1, Z and Z + 1 are forming...
Text Solution
|
- Identify the molecule in which the arrangement of electron pairs aroun...
Text Solution
|
- The wave functions of Is-orbitals of two hydrogen atoms are Psi(A) " ...
Text Solution
|
- The variation of vapour pressure (b) as a function of temperature (a) ...
Text Solution
|
- Which one of the following contains - As = As in its structure ?
Text Solution
|
- What are X and Y in the following reaction sequence ?
Text Solution
|
- Which of the following sets is in the correct order regerding the prop...
Text Solution
|
- Identify the products (X,Y) and reaction mechanism (Z) of the followin...
Text Solution
|
- What are the products formed when an aldehyde (RCHO) is reacted with T...
Text Solution
|
- The following species are involved in the formation of an ester from a...
Text Solution
|
- Identify the reagents (X,Y,Z) used in the conversion of 3-methylanilin...
Text Solution
|