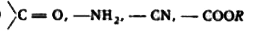
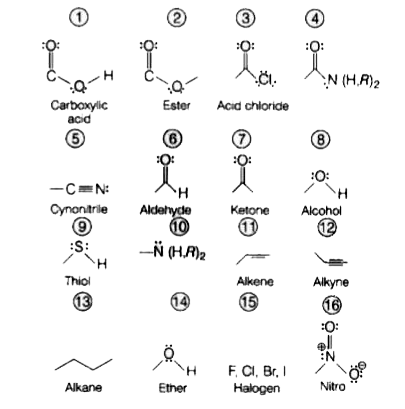
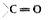A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- T he order of priority of the following functional group iin IUPAG met...
Text Solution
|
- The decreasing order of priority of the following functional group is:...
Text Solution
|
- The decreasing order of priority for the following functional group is...
Text Solution
|
- The decreasig order of priority for the following functional groups is...
Text Solution
|
- The correct IUPAG name of following compound is:
Text Solution
|
- The correct order priority for the -CONH(2)CN" and "-COOR is
Text Solution
|
- Decreasing -I effect of given groups is : (i) -CN" (ii)"-NO(2...
Text Solution
|
- What are the compounds containing the -COOR group as the functional gr...
Text Solution
|
- T he order of priority of the following functional group iin IUPAG met...
Text Solution
|