A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-AP EAMCET SOLVED PAPER 2019 (22-04-2019, SHIFT-2)-CHEMISTRY
- An element forms a body centered cubic (bcc) lattice with edge length ...
Text Solution
|
- An ideal solution of hexane and heptane at 30^(@)C has a vapour pressu...
Text Solution
|
- The vapour pressure of a solution (b) as a function of temperature (a)...
Text Solution
|
- If E(cell)^(@) is 1.05 V, the emf of the cell for the following cell r...
Text Solution
|
- Which one of the following is not the correct statement with respect t...
Text Solution
|
- 300 mL of gold sol is mixed with 30 mL of 10% NaCl solution. The mass ...
Text Solution
|
- In froth-floatation process, what is the depressant used in the separa...
Text Solution
|
- In which of the following oxyacids of phosphorus, one P = 0, two P-H n...
Text Solution
|
- Identify the reaction in which SO(2) is not formed?
Text Solution
|
- In which of the following reactions oxygen gas is not formed?
Text Solution
|
- The magnetic moment of which of the following complexes is maximum?
Text Solution
|
- A metal ions (M^(n+)) forms octahedral [ML(6)]^(n+) and tetrahedral [M...
Text Solution
|
- The catalyst triethyl aluminium and titanium tetrachloride finds use i...
Text Solution
|
- Match the following :
Text Solution
|
- Identify the correct pairs from the following :
Text Solution
|
- The number of monochloroderivatives possible, when 2, 2-dimethylpropan...
Text Solution
|
- In the following sequence of reactions identify the functional groups ...
Text Solution
|
- The correct set of reagents (X, Y, Z) required to convert benzene to m...
Text Solution
|
- The reduction products of an aldehyde, ketone and carboxylic acid in t...
Text Solution
|
- A carbonyl compound A (C(8)H(8)O) does not give iodoform test and on o...
Text Solution
|
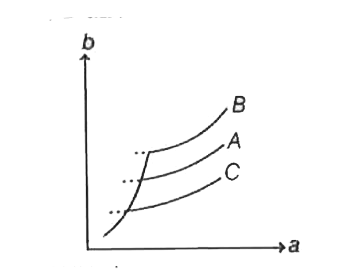
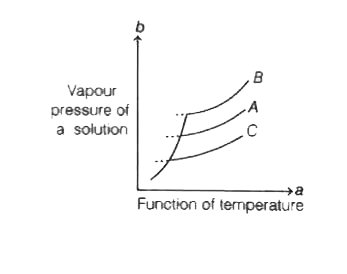 When number of moles of solute decreases, then vapour pressure of solution increses.
When number of moles of solute decreases, then vapour pressure of solution increses.