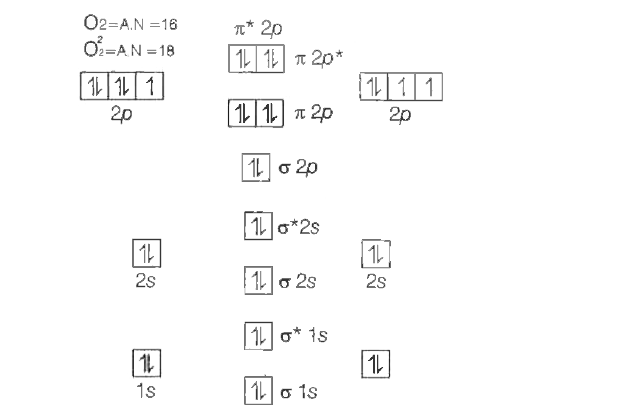
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The number of electrons present in bonding and antibonding orbitals in...
Text Solution
|
- The number of antibonding electron pairs in O(2)^(2-) molecular ion on...
Text Solution
|
- Number of antibonding electrons in O(2)^(-) molecular ion is :
Text Solution
|
- Total number of molecular orbitals occupying one or two electrons in O...
Text Solution
|
- The number of antibonding electron pairs in O(2)^(2-) molecular ion on...
Text Solution
|
- Total number of antibonding electrons present in O(2) will be-
Text Solution
|
- The bond order depends on the number of electrons in the bonding and a...
Text Solution
|
- According to molecular orbital theory molecules are said to be stable ...
Text Solution
|
- The number of antibonding electron pairs in O(2)^(-)
Text Solution
|