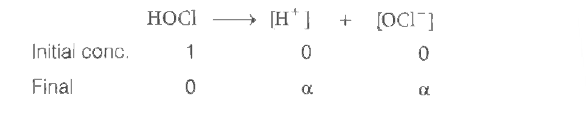A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- If the ionization constant of hypochlorous acid (HOCl) is 2.5xx10^(-5)...
Text Solution
|
- The ionization constant of propanoic acid is 1.32xx10^(-5). Calculate ...
Text Solution
|
- The ionization constant of propionic acid is 1.32xx10^(-5) . Calculate...
Text Solution
|
- An acid HA ionizes as HAhArrH^(+)+A^(-) The pH of 1.0 M solution is 5....
Text Solution
|
- Calculate the pH of 0.08 solution of HOCI (hydrochlorous acid). The io...
Text Solution
|
- The pH of 0.1 M solution of cyanic acid (HCNO) is 2.34. Calculate the ...
Text Solution
|
- 0.08 हाइपोक्लोरस अम्ल (HOCl ) के विलयन के pH की गणना कीजिए । ...
Text Solution
|
- The acid ionization (hydrolysis) constant of Zn^(2+)" is "1.0 xx 10^(-...
Text Solution
|
- If the ionization constant of hypochlorous acid (HOCl) is 2.5xx10^(-5)...
Text Solution
|