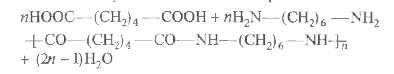A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Nylon 6, 6 is a condensation polymer of two monomers X and Y. The numb...
Text Solution
|
- How many functional groups are present in monomer of polymer nylon-6?
Text Solution
|
- नायलॉन-6, 6…………….तथा………..का संघनन बहुलक है।
Text Solution
|
- The monomers of biodegradable polymer , nylon 2-nylon 6 are
Text Solution
|
- The monomer units of Nylon 6,6 Nylon 2-Nylon 6 are respectively,
Text Solution
|
- Identify the monomers used in the manufacture of glyptal (X), dacron (...
Text Solution
|
- Condensation polymers| polyamides (Nylon 6|6; Nylon 6; Nylon 2| 6)| po...
Text Solution
|
- Nylon 6, 6 is a condensation polymer of two monomers X and Y. The numb...
Text Solution
|
- Consider following polymers: " Polythene ,Bakelite, Nylon 6-6, Dacron,...
Text Solution
|