Similar Questions
Explore conceptually related problems
Recommended Questions
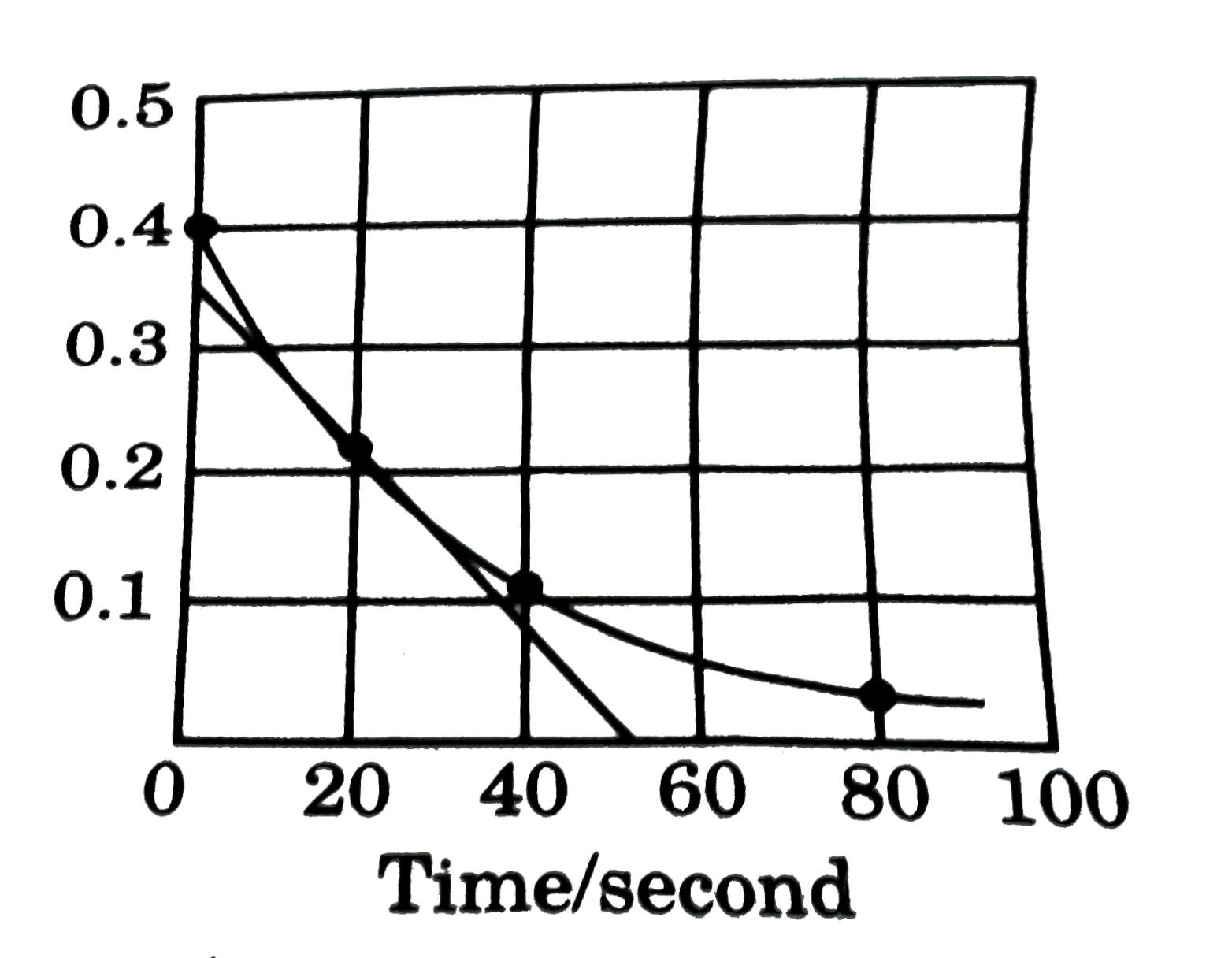
- A reaction follows the given concentration (M) vs time graph. The rate...
Text Solution
|
- The rate constant of the reaction A rarr B is 0.6 xx 10^(-3) mole per ...
Text Solution
|
- A reaction follows the given concentration (M) vs time graph. The rate...
Text Solution
|
- If the graph of concentration of [A] vs T for completion of reaction ...
Text Solution
|
- For a general reaction A to B. plot of concentrating of A vs time is g...
Text Solution
|
- For a general reaction A to B, plot of concentration of A vs time ...
Text Solution
|
- Which among the following plots are linear (a -x) is the concentration...
Text Solution
|
- In which of the following reactions the graph of concentration vs time...
Text Solution
|
- plot a graph of reaction -rate vs concentration of the reactant for a ...
Text Solution
|